
Which one of the following is dibasic acid?
\[
A.\;\;\;\;\;{H_3}P{O_4} \\
B.\;\;\;\;\;{H_3}P{O_3} \\
C.\;\;\;\;\;{H_3}B{O_3} \\
D.\;\;\;\;\;{H_3}As{O_4} \\
\]
Answer
507.3k+ views
Hint: draw the structures of all the compounds and count the number of hydrogens linked to the oxygen atoms. Only ${H_3}P{O_3}$ is dibasic; all the others are tribasic. Hydrogens linked directly to the electronegative atom cannot be released as ${H^ + }$ ions.
Complete answer:
Dibasic acids are acids that can donate two protons or acids containing two replaceable hydrogen atoms. They are also called diprotic acids. Some of its examples are Sulphuric acid, carbonic acid, oxalic acid. when dissociation of dibasic takes place it occurs in two steps. It has two dissociation constants.
In the above question we are given some compounds and we have to identify which one among them is a dibasic acid. For this we will look into the structures of each of the options.
The structure of the compounds are as follows:
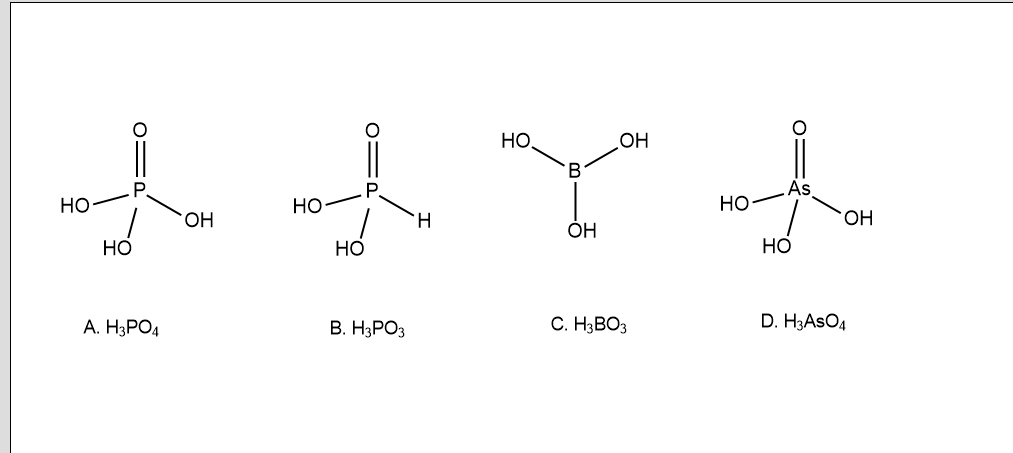
To identify which among them is dibasic, it is important to note that only those hydrogen ions will be replaced in aqueous state which is linked with the Oxygen atom. If the hydrogen atom is linked with the Phosphorus atom then there won't be any loss of such a hydrogen atom.
Taking this into consideration, if we observe the above image we see that all the compounds except ${H_3}P{O_3}$ have three hydrogen atoms which are linked to three oxygen atoms. This means that in these compounds three hydrogen ions will be replaceable. Therefore these compounds can be called tribasic acids. In the above image, ${H_3}P{O_3}$ has two hydrogens linked to two oxygen atoms, and one hydrogen is linked to phosphorus thus hydrogens linked to oxygen will be replaceable and hydrogen linked to phosphorus cannot be replaced. Thus ${H_3}P{O_3}$ is a dibasic acid whereas all others are tribasic acids.
So, the correct answer is “Option B”.
Note:
The number of hydrogen present in the compound does not give the basicity of an acid. if in the compounds hydrogens are linked to oxygen atoms only then they will be considered as basic acids. If the hydrogen atom is directly linked the electronegative atoms are not replaceable. There are also monobasic and polybasic acids. In the acidity of bases, the number of hydroxyl ions that the basic molecule can produce in an aqueous solution is calculated.
Complete answer:
Dibasic acids are acids that can donate two protons or acids containing two replaceable hydrogen atoms. They are also called diprotic acids. Some of its examples are Sulphuric acid, carbonic acid, oxalic acid. when dissociation of dibasic takes place it occurs in two steps. It has two dissociation constants.
In the above question we are given some compounds and we have to identify which one among them is a dibasic acid. For this we will look into the structures of each of the options.
The structure of the compounds are as follows:

To identify which among them is dibasic, it is important to note that only those hydrogen ions will be replaced in aqueous state which is linked with the Oxygen atom. If the hydrogen atom is linked with the Phosphorus atom then there won't be any loss of such a hydrogen atom.
Taking this into consideration, if we observe the above image we see that all the compounds except ${H_3}P{O_3}$ have three hydrogen atoms which are linked to three oxygen atoms. This means that in these compounds three hydrogen ions will be replaceable. Therefore these compounds can be called tribasic acids. In the above image, ${H_3}P{O_3}$ has two hydrogens linked to two oxygen atoms, and one hydrogen is linked to phosphorus thus hydrogens linked to oxygen will be replaceable and hydrogen linked to phosphorus cannot be replaced. Thus ${H_3}P{O_3}$ is a dibasic acid whereas all others are tribasic acids.
So, the correct answer is “Option B”.
Note:
The number of hydrogen present in the compound does not give the basicity of an acid. if in the compounds hydrogens are linked to oxygen atoms only then they will be considered as basic acids. If the hydrogen atom is directly linked the electronegative atoms are not replaceable. There are also monobasic and polybasic acids. In the acidity of bases, the number of hydroxyl ions that the basic molecule can produce in an aqueous solution is calculated.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




