
Which one of the following is reducing sugar?
(a) Glucose
(b) Starch
(c) Cellulose
(d) Sucrose
Answer
524.8k+ views
Hint: This is made naturally by green plants and some photosynthetic forms of algae and is considered to be the most abundant form of a monosaccharide sugar.
Complete answer:
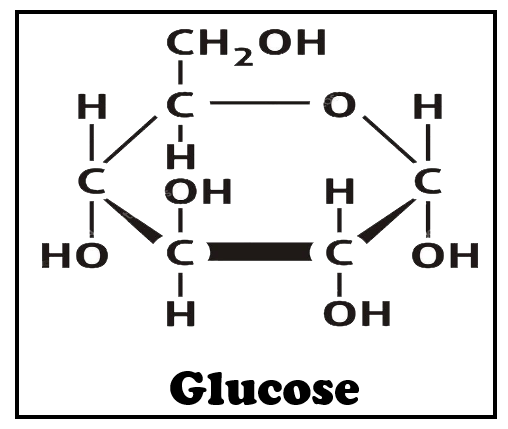
Any sugar is said to be a reducing sugar if it is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. Along with some disaccharides, some oligosaccharides, and some polysaccharides, all monosaccharides are reducing sugars. Glucose has a free aldehyde group which can be oxidized to the acidic groups. Hence, glucose is a reducing sugar. The glucose in starch and cellulose doesn't contain a free aldehyde radical and hence, starch and cellulose don't act as reducing sugars.
Similarly, sucrose may be a disaccharide during which the aldehyde radical of glucose is employed up in forming a bond between glucose and fructose.
Additional Information:
-Reducing sugar is any sugar that's capable of acting as a reducer because it's a free aldehyde radical or a free group.
-All monosaccharides are reducing sugars, alongside some disaccharides, some oligosaccharides, and a few polysaccharides.
-The monosaccharides are often divided into two groups: the aldoses, which have an aldehyde radical, and therefore the ketoses, which have a group. Ketoses must first tautomerize to aldoses before they will act as reducing sugars. Galactose, glucose, and fructose are all reducing sugars and also common dietary monosaccharides.
-Disaccharides are formed from two monosaccharides and may be classified as either reducing or nonreducing. The glycosidic bonds are present in the nonreducing disaccharides like sucrose and trehalose.
-The bond is present between their anomeric carbons and thus unable to convert to an open-chain form with an aldehyde group; in which they're stuck in the cyclic form.
-Reducing disaccharides like lactose and maltose have just one of their two anomeric carbons involved within the glycosidic bond, while the opposite is free and may convert to an open-chain form with an aldehyde group.
Note: -The aldehyde functional group allows the sugar to act as a reducer, for instance, within the Tollens' test or Benedict's test. The cyclic hemiacetal sorts of aldoses can hospitably reveal an aldehyde, and certain ketoses can undergo tautomerization to become aldoses. However, the acetals, including those found in the polysaccharide linkages, cannot easily become free aldehydes.
-Reducing sugars react with amino acids within the Maillard reaction, a series of reactions that happens while cooking food at high temperatures which is vital in determining the flavor of food.
- Also, the amount of reducing sugars in wine, juice, and sugarcane are indicative of the standard of those food products.
Complete answer:
Any sugar is said to be a reducing sugar if it is capable of acting as a reducing agent because it has a free aldehyde group or a free ketone group. Along with some disaccharides, some oligosaccharides, and some polysaccharides, all monosaccharides are reducing sugars. Glucose has a free aldehyde group which can be oxidized to the acidic groups. Hence, glucose is a reducing sugar. The glucose in starch and cellulose doesn't contain a free aldehyde radical and hence, starch and cellulose don't act as reducing sugars.
Similarly, sucrose may be a disaccharide during which the aldehyde radical of glucose is employed up in forming a bond between glucose and fructose.

Additional Information:
-Reducing sugar is any sugar that's capable of acting as a reducer because it's a free aldehyde radical or a free group.
-All monosaccharides are reducing sugars, alongside some disaccharides, some oligosaccharides, and a few polysaccharides.
-The monosaccharides are often divided into two groups: the aldoses, which have an aldehyde radical, and therefore the ketoses, which have a group. Ketoses must first tautomerize to aldoses before they will act as reducing sugars. Galactose, glucose, and fructose are all reducing sugars and also common dietary monosaccharides.
-Disaccharides are formed from two monosaccharides and may be classified as either reducing or nonreducing. The glycosidic bonds are present in the nonreducing disaccharides like sucrose and trehalose.
-The bond is present between their anomeric carbons and thus unable to convert to an open-chain form with an aldehyde group; in which they're stuck in the cyclic form.
-Reducing disaccharides like lactose and maltose have just one of their two anomeric carbons involved within the glycosidic bond, while the opposite is free and may convert to an open-chain form with an aldehyde group.
Note: -The aldehyde functional group allows the sugar to act as a reducer, for instance, within the Tollens' test or Benedict's test. The cyclic hemiacetal sorts of aldoses can hospitably reveal an aldehyde, and certain ketoses can undergo tautomerization to become aldoses. However, the acetals, including those found in the polysaccharide linkages, cannot easily become free aldehydes.
-Reducing sugars react with amino acids within the Maillard reaction, a series of reactions that happens while cooking food at high temperatures which is vital in determining the flavor of food.
- Also, the amount of reducing sugars in wine, juice, and sugarcane are indicative of the standard of those food products.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




