
Which pair of statements about diamond and graphite is correct?
A.Diamond and graphite are both pure carbons. They are both macromolecules.
B.Diamond and graphite can both be used as electrodes. Graphite is also used as a lubricant.
C.Diamond has covalent bonds. Graphite has ionic bonds.
D.Diamond is hard with a high melting point. Graphite is soft with a low melting point.
Answer
579.6k+ views
Hint: Allotropy is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Diamond, Graphite and Fullerene are popular allotropes of Carbon. Diamond is the hardest substance in the world. Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure.
Complete step by step answer:
Graphite is the most stable allotrope of carbon. Under normal conditions, the diamond is metastable, meaning that it converts back to graphite when the process is initiated with sufficient energy. All allotrope of carbon turns into graphite at higher temperatures. Diamond also turns into graphite at a temperature between $1800 - {2000^ \circ }C$ .
Diamond cannot conduct electricity. On the other hand, graphite can conduct electricity. Therefore, the diamond cannot be used as an electrode. Graphite and carbon both are macromolecules of carbon only with a different arrangement of carbon molecules. In graphite and diamond both have covalent bonds. Both graphite and diamond have the boiling point at \[{3600^\circ }C\] . The structures are shown below,
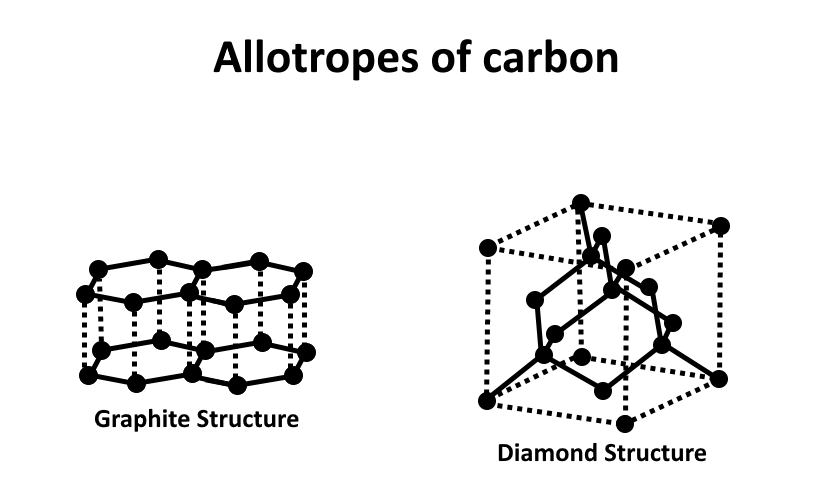
So, the correct option is, A.
Note: Diamond is harder than graphite because, in diamond, each carbon atom forms 4 covalent bonds in a tetrahedral structure. The sheets of carbon become bonded by weaker intermolecular forces. It is because of these weak intermolecular forces that the layers of graphite can slide over each other, making the overall substance a lot weaker than diamond.
Complete step by step answer:
Graphite is the most stable allotrope of carbon. Under normal conditions, the diamond is metastable, meaning that it converts back to graphite when the process is initiated with sufficient energy. All allotrope of carbon turns into graphite at higher temperatures. Diamond also turns into graphite at a temperature between $1800 - {2000^ \circ }C$ .
Diamond cannot conduct electricity. On the other hand, graphite can conduct electricity. Therefore, the diamond cannot be used as an electrode. Graphite and carbon both are macromolecules of carbon only with a different arrangement of carbon molecules. In graphite and diamond both have covalent bonds. Both graphite and diamond have the boiling point at \[{3600^\circ }C\] . The structures are shown below,

So, the correct option is, A.
Note: Diamond is harder than graphite because, in diamond, each carbon atom forms 4 covalent bonds in a tetrahedral structure. The sheets of carbon become bonded by weaker intermolecular forces. It is because of these weak intermolecular forces that the layers of graphite can slide over each other, making the overall substance a lot weaker than diamond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




