
Which plots represents an exothermic reaction?
A.
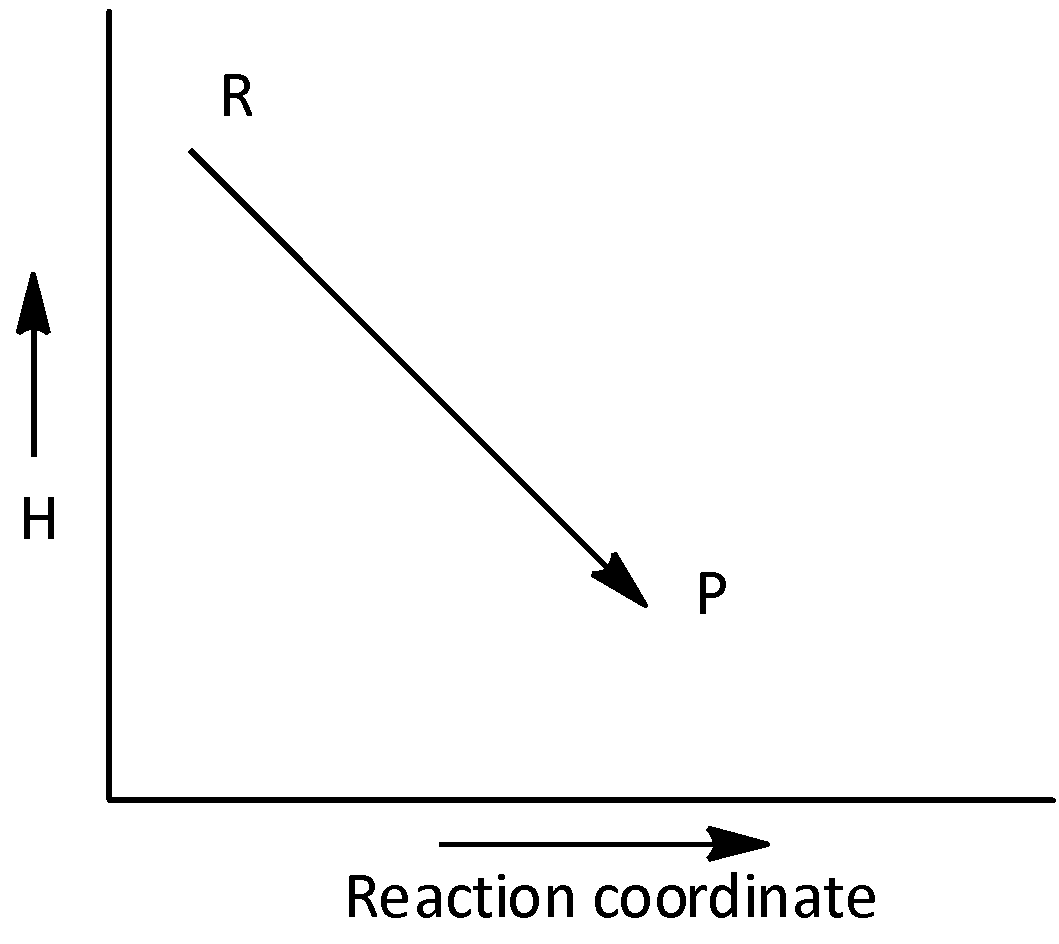
B.
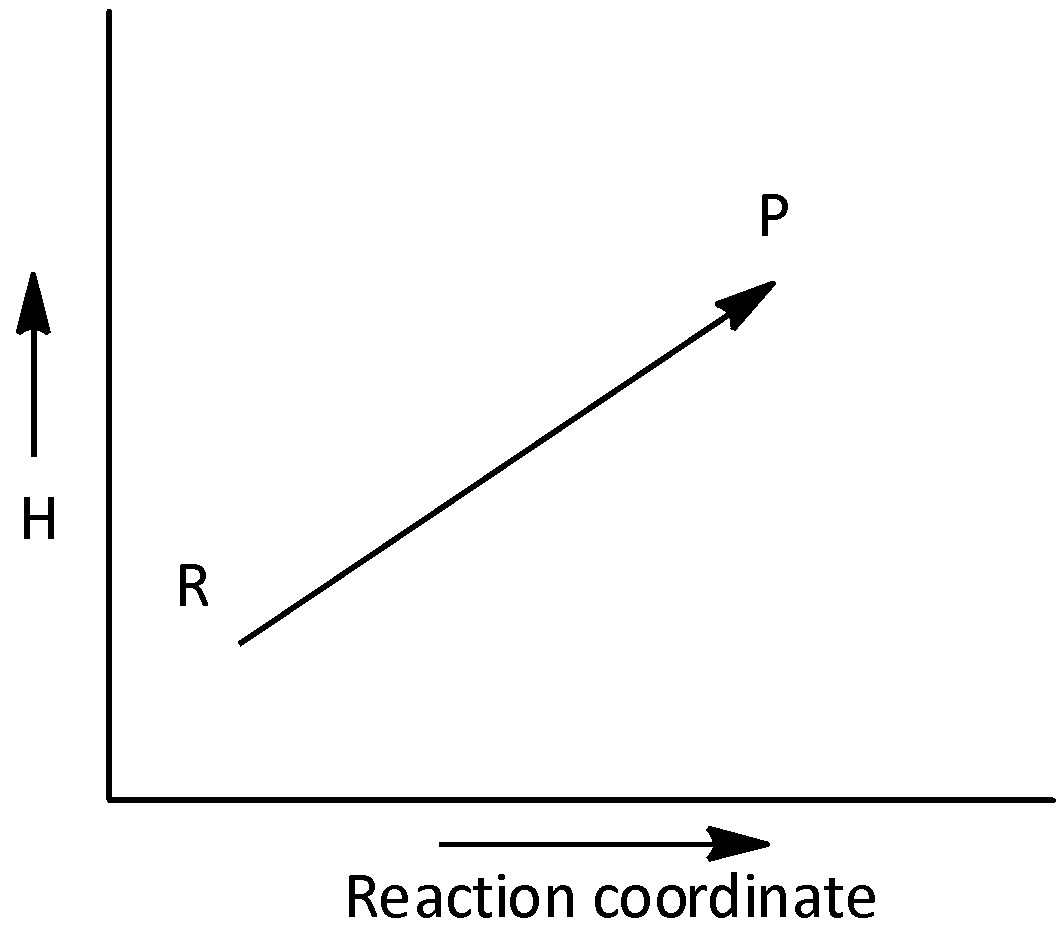
C.
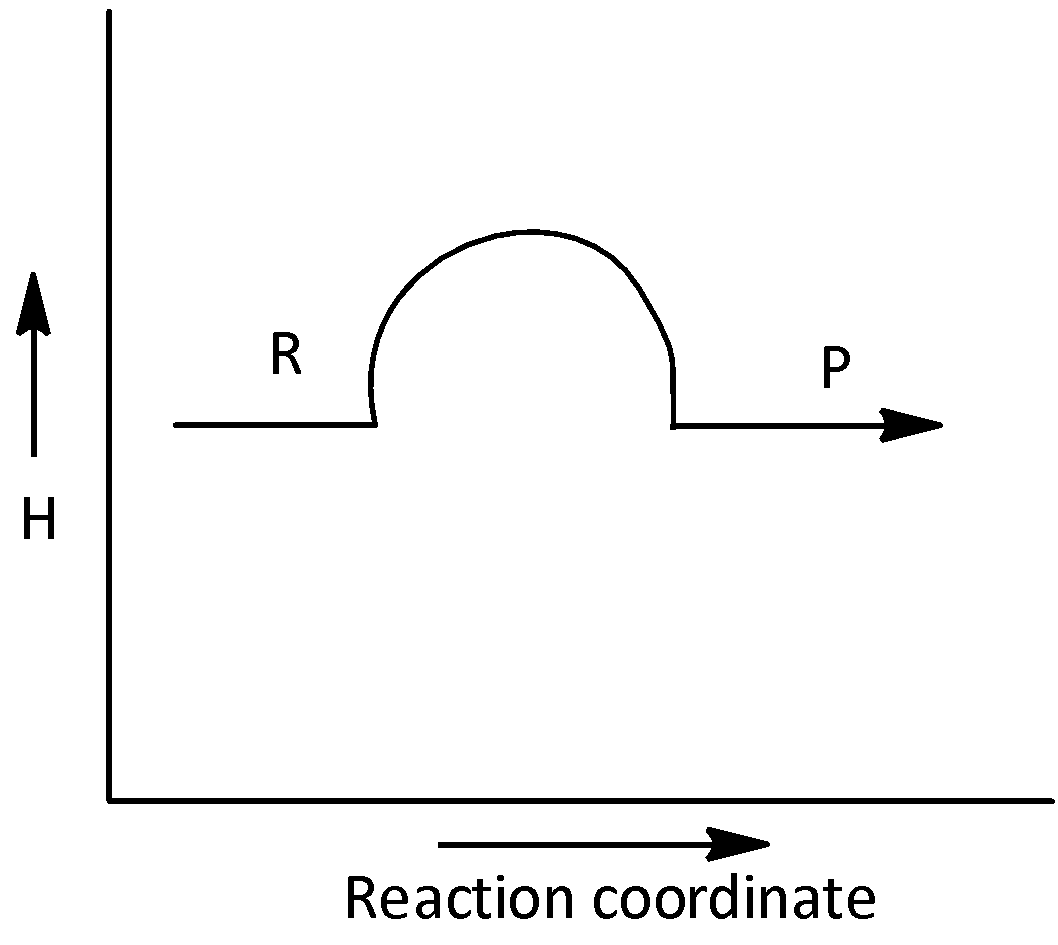
D.





Answer
495.6k+ views
Hint: We need to know that during a chemical reaction, the reactants are converted into products. Maybe, one or more reactants are present and It will be transformed into more different products and that substance is known as products. In the case of an exothermic reaction, the heat will release from the system to the surrounding area during the reaction and the enthalpy change of the reaction becomes negative. But in the case of endothermic reaction, the reactant will absorb the heat and there is a formation of product.
Complete answer:
The given plot represents the exothermic reaction. Let’s see the graph-
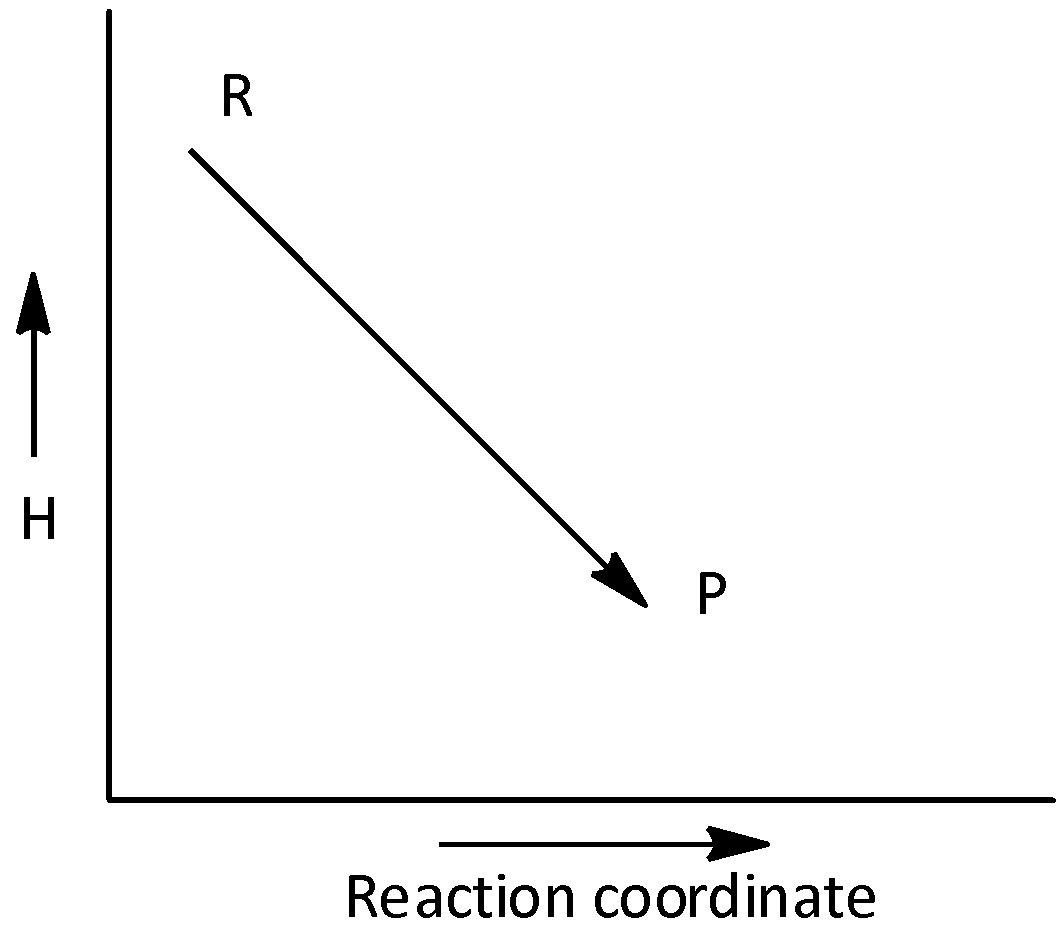
Here, the graph plot between enthalpy, (H) and reaction coordinate. And in the case of exothermic reaction, the enthalpy change is greater than zero, \[\Delta H < 0\]
Thus, the enthalpy change of the reaction is equal to subtraction of enthalpy of reaction from enthalpy of product which is equal to greater than zero.
\[\Delta H = {H_{product}} - {H_{reac\tan t}} < 0\]
That is, the enthalpy of product is greater than the enthalpy of the reactant.
\[{H_{product}} < {H_{reac\tan t}}\]
Hence, option (A) is correct.
The given graph does not represents an exothermic reaction. Hence, option (B) is incorrect.
The given graph does not represents an exothermic reaction. Hence, option (C) is incorrect.
The given graph does not represents an exothermic reaction. Hence, option (D) is incorrect.
Hence, option (A) is correct.
Note:
We know that in the case of exothermic reaction, the system eliminates the heat and to the surroundings from the system. And the sum of enthalpy of reactant is higher than the enthalpy of product. If the products contain greater enthalpy, then the reactions are endothermic. And in an endothermic reaction, the enthalpy change is positive.
Complete answer:
The given plot represents the exothermic reaction. Let’s see the graph-

Here, the graph plot between enthalpy, (H) and reaction coordinate. And in the case of exothermic reaction, the enthalpy change is greater than zero, \[\Delta H < 0\]
Thus, the enthalpy change of the reaction is equal to subtraction of enthalpy of reaction from enthalpy of product which is equal to greater than zero.
\[\Delta H = {H_{product}} - {H_{reac\tan t}} < 0\]
That is, the enthalpy of product is greater than the enthalpy of the reactant.
\[{H_{product}} < {H_{reac\tan t}}\]
Hence, option (A) is correct.
The given graph does not represents an exothermic reaction. Hence, option (B) is incorrect.
The given graph does not represents an exothermic reaction. Hence, option (C) is incorrect.
The given graph does not represents an exothermic reaction. Hence, option (D) is incorrect.
Hence, option (A) is correct.
Note:
We know that in the case of exothermic reaction, the system eliminates the heat and to the surroundings from the system. And the sum of enthalpy of reactant is higher than the enthalpy of product. If the products contain greater enthalpy, then the reactions are endothermic. And in an endothermic reaction, the enthalpy change is positive.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




