
Which points on the potential energy diagram represent the eclipsed conformation of ethane and staggered conformation of propane respectively:
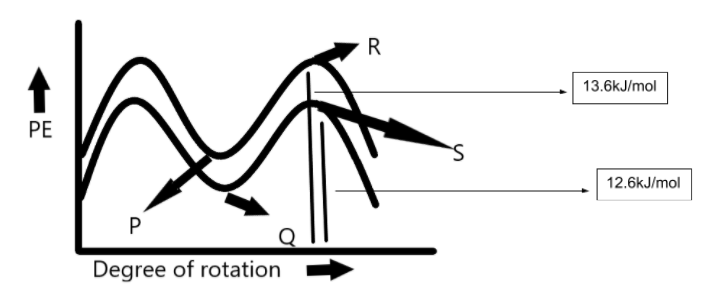
A.P, R
B.Q, R
C.S, P
D.R, Q

Answer
573.9k+ views
Hint: To answer this question, you should recall the concept of Conformational isomerism. It involves rotation about sigma bonds and does not involve any differences in the connectivity of the atoms or geometry of bonding. Two or more structures that are categorized as conformational isomers, or conformers, are just two of the same molecules that differ only in the rotation of one or more sigma bonds.
Complete step by step answer:
Alkanes can thus have an infinite number of conformations by rotation around carbon bonds. However, this rotation is not completely free due to repulsive interactions between the electron clouds of carbon-hydrogen bonds. This repulsive interaction is termed as torsional strain. The eclipsed conformation of ethane is represented by point S. This conformation has maximum potential energy but it is lower than the eclipsed conformation of propane. The staggered conformation of propane is represented by point P. It has the lowest potential energy but it is higher than the potential energy of staggered conformation of ethane.
Propane has more conformational energy as compared to ethane. Eclipsed conformation of ethane has more Vander Val strain and torsional strain so it lies to the upper side in the P.E. diagram and staggered conformation of propane has less Vander Val strain and torsional strain so it lies to the lower side in P.E. diagram.
Hence, the correct answer to this question is option C.
Note:
You should note that unhindered rotations do not exist in Ethane because the carbon-carbon bond is not completely free to rotate due to the torsional strain in ethane creating a barrier to the rotation that must be overcome for the bond to rotate from one staggered conformation to another. This rotational barrier is not large enough to prevent rotation except at extremely cold temperatures.
Complete step by step answer:
Alkanes can thus have an infinite number of conformations by rotation around carbon bonds. However, this rotation is not completely free due to repulsive interactions between the electron clouds of carbon-hydrogen bonds. This repulsive interaction is termed as torsional strain. The eclipsed conformation of ethane is represented by point S. This conformation has maximum potential energy but it is lower than the eclipsed conformation of propane. The staggered conformation of propane is represented by point P. It has the lowest potential energy but it is higher than the potential energy of staggered conformation of ethane.
Propane has more conformational energy as compared to ethane. Eclipsed conformation of ethane has more Vander Val strain and torsional strain so it lies to the upper side in the P.E. diagram and staggered conformation of propane has less Vander Val strain and torsional strain so it lies to the lower side in P.E. diagram.
Hence, the correct answer to this question is option C.
Note:
You should note that unhindered rotations do not exist in Ethane because the carbon-carbon bond is not completely free to rotate due to the torsional strain in ethane creating a barrier to the rotation that must be overcome for the bond to rotate from one staggered conformation to another. This rotational barrier is not large enough to prevent rotation except at extremely cold temperatures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




