
Which substance changes its colour from orange to pink in acidic solution?
A. Litmus paper
B. Methyl orange
C. Phenolphthalein
D. None of the above
Answer
514.2k+ views
Hint : The chemical indicator is a substance which when comes in contact with a solution, changes its colour to indicate whether the solution is acidic or basic. Chemical indicators are divided into two types i.e., Artificial indicators like phenolphthalein, methyl orange etc. and Natural indicators like litmus, turmeric, beetroot, etc.
Complete Step By Step Answer:
The changes in colour of the given indicators in the acidic solution are as follows:
Litmus paper:
Litmus is the most commonly used natural indicator which is obtained from lichens. It is usually found in the form of strips of paper known as litmus paper. The litmus paper is found in two colours i.e., red and blue. On testing litmus paper with an acidic solution, it turns blue litmus paper into red.
Methyl orange:
It is an artificial indicator which is frequently used in titrations because of its specific and clear colour variance at different values of pH. On using methyl orange in acidic medium, its colour changes from orange to pink colour. The chemical structure of methyl orange is as follows:
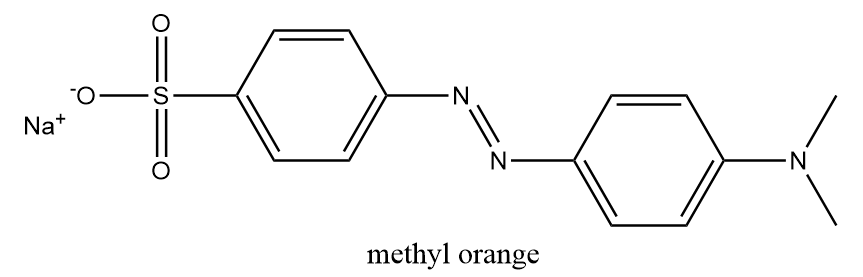
Phenolphthalein:
It is an organic compound and an artificial indicator which is used as an indicator in acid-base titrations. It is originally colourless and no change in its colour is observed when tested for acidic solutions i.e., it remains colourless in acidic solutions. The chemical structure of phenolphthalein is as follows:
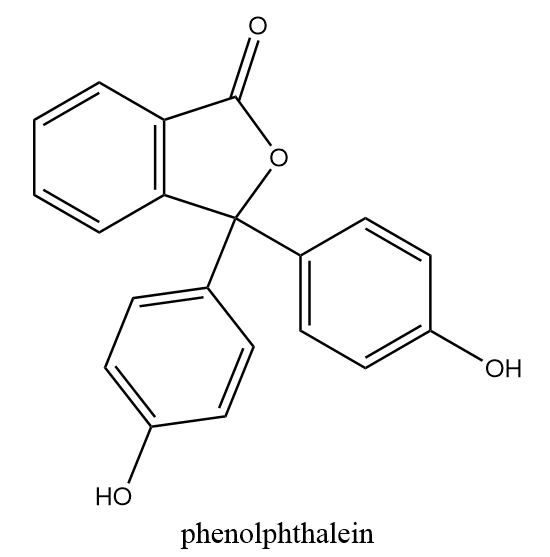
Hence, methyl orange is the indicator which changes its colour from orange to pink in acidic solutions.
So, the correct answer is option (B).
Note :
It is important to note that, in basic solution the changes observed in the given indicators are as follows:
-Litmus- In basic medium, it turns red litmus paper into blue.
-Methyl orange- In basic medium, its colour changes from orange to yellow.
-Phenolphthalein- In basic medium, it changes from colourless to pink colour.
Complete Step By Step Answer:
The changes in colour of the given indicators in the acidic solution are as follows:
Litmus paper:
Litmus is the most commonly used natural indicator which is obtained from lichens. It is usually found in the form of strips of paper known as litmus paper. The litmus paper is found in two colours i.e., red and blue. On testing litmus paper with an acidic solution, it turns blue litmus paper into red.
Methyl orange:
It is an artificial indicator which is frequently used in titrations because of its specific and clear colour variance at different values of pH. On using methyl orange in acidic medium, its colour changes from orange to pink colour. The chemical structure of methyl orange is as follows:

Phenolphthalein:
It is an organic compound and an artificial indicator which is used as an indicator in acid-base titrations. It is originally colourless and no change in its colour is observed when tested for acidic solutions i.e., it remains colourless in acidic solutions. The chemical structure of phenolphthalein is as follows:

Hence, methyl orange is the indicator which changes its colour from orange to pink in acidic solutions.
So, the correct answer is option (B).
Note :
It is important to note that, in basic solution the changes observed in the given indicators are as follows:
-Litmus- In basic medium, it turns red litmus paper into blue.
-Methyl orange- In basic medium, its colour changes from orange to yellow.
-Phenolphthalein- In basic medium, it changes from colourless to pink colour.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




