
Which type of shape is found in $S{{F}_{2}}$ molecule?
(A) V-shaped
(B) Bipyramidal
(C) Liner
(D) irregular tetrahedron
Answer
584.7k+ views
Hint: Lewis's concept is unable to explain the shapes of the molecule. The Valence shell electron pair repulsion theory provides a simple procedure to predict the shapes of covalent molecules. This theory is based on the repulsive interactions of electron pairs in the valence shell of the atom.
Complete step by step solution:
According to VSEPR theory, the valence electron pairs in a molecule will arrange themselves around the central atoms of the molecule and so that they are as far apart as they can be.
Assumptions of VSEPR theory:
The unpaired electrons in the valence shell of the central atom form a bond with the unpaired electron of surrounding atoms, whereas paired electrons remain as lone pairs in the valence shell of the central atom.
The electrons in the orbital of the valence shell of the central atom should be arranged in a space such that hybrid orbitals lie as far away from one another to give maximum stability of the molecule.
Lone pair and bond pair are repelling each other.
The number of bond pairs as well as lone pairs surrounded by the central atom decides the geometry and shape of the molecule.
Electron pairs around the central atom should adjust themselves to reduce the minimum repulsive forces around the central atom.
There are three types of repulsion, lp-lp, lp-bp, bp-bp. The magnitude of repulsive forces are different as follows: lp-lp>lp-bp>bp-bp
Given sulfur difluoride molecule shape will be predicted by using this VSEPR theory.
The central atom is S, its atomic number is 16, and electronic configuration is - $[Ne]3{{s}^{2}}3{{p}^{4}}$
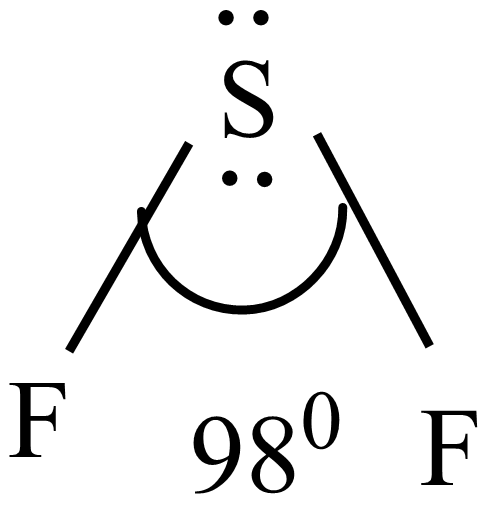
When sulfur forms three hybrid four hybrid orbitals of $s{{p}^{3}}$ hybridization. Out of four $s{{p}^{3}}$hybrid orbitals, two orbitals are filled with lone pairs, and the remaining two are with one electron. Even though the structure is tetrahedral due to $s{{p}^{3}}$hybridization. But the structure became distorted tetrahedral and exists to V-shaped due to lone pair-bond pair repulsion around the central atom. The bond angle F-S-F is ${{98}^{0}}$ and structure is the bent shape or V-shape.
The correct answer is option A.
Note: Determining the molecule interactions and reactions with other molecules, its shape is important, which also influences the boiling point and melting point of molecules. The property of molecules depends on a particular shape and form this shape able to define many properties.
Complete step by step solution:
According to VSEPR theory, the valence electron pairs in a molecule will arrange themselves around the central atoms of the molecule and so that they are as far apart as they can be.
Assumptions of VSEPR theory:
The unpaired electrons in the valence shell of the central atom form a bond with the unpaired electron of surrounding atoms, whereas paired electrons remain as lone pairs in the valence shell of the central atom.
The electrons in the orbital of the valence shell of the central atom should be arranged in a space such that hybrid orbitals lie as far away from one another to give maximum stability of the molecule.
Lone pair and bond pair are repelling each other.
The number of bond pairs as well as lone pairs surrounded by the central atom decides the geometry and shape of the molecule.
Electron pairs around the central atom should adjust themselves to reduce the minimum repulsive forces around the central atom.
There are three types of repulsion, lp-lp, lp-bp, bp-bp. The magnitude of repulsive forces are different as follows: lp-lp>lp-bp>bp-bp
Given sulfur difluoride molecule shape will be predicted by using this VSEPR theory.
The central atom is S, its atomic number is 16, and electronic configuration is - $[Ne]3{{s}^{2}}3{{p}^{4}}$
When sulfur forms three hybrid four hybrid orbitals of $s{{p}^{3}}$ hybridization. Out of four $s{{p}^{3}}$hybrid orbitals, two orbitals are filled with lone pairs, and the remaining two are with one electron. Even though the structure is tetrahedral due to $s{{p}^{3}}$hybridization. But the structure became distorted tetrahedral and exists to V-shaped due to lone pair-bond pair repulsion around the central atom. The bond angle F-S-F is ${{98}^{0}}$ and structure is the bent shape or V-shape.

The correct answer is option A.
Note: Determining the molecule interactions and reactions with other molecules, its shape is important, which also influences the boiling point and melting point of molecules. The property of molecules depends on a particular shape and form this shape able to define many properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




