
Which will form geometrical isomers.
(A)
(B)
(C)
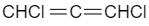
(D) Both a and b


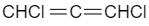
Answer
512.1k+ views
Hint :The total number of ligands attached to a central metal atom or ion is called the coordination number of that ion.
Geometrical isomerism is not shown by complexes with coordination number 2 and 3, square planar complexes of the type $ M{A_4},M{A_3}B,MA{B_3} $ and octahedral complexes of $ M{A_6},M{A_5}B $ type.
Complete Step By Step Answer:
Geometrical isomerism arises in heteroleptic complexes due to ligands occupying different positions around the central ion. The ligands occupy positions either adjacent to one another or opposite to one another. If the ligands occupy adjacent positions they are referred as cis-form and if the ligands occupies opposite positions they are referred as trans-form.
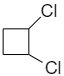
1,2-dichlorocyclobutane shows geometrical isomerism because it forms cis-1,2-dichlorocyclobutane and trans-1,2-dichlorocyclobutane as shown below

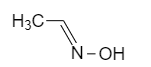
Oximes or N-hydroxy alkane mines also shows geometrical isomerism.
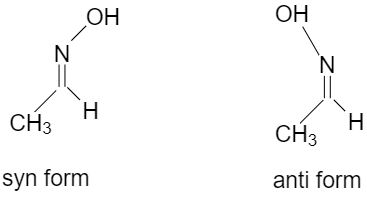
syn oxime is the one which has both hydrogen and hydroxyl groups attached to the $ C = N $ side and in anti oxime the hydrogen and hydroxyl group attached to the opposite of the $ C = N $ $ C = N $ side.
As the attached groups are present in different planes so 1,3-dichloroallene does not show geometrical isomerism.
So the correct answer for the question is option D. 1,2 dichlorocyclobutane and oxime will form geometrical isomers.
Note :
The mirror images compounds are non-superimposable on each other and do not possess the plane of symmetry. The optical isomers also possess the property of chirality.
The essential condition for a substance to show optical activity is that the substance should not have a plane of symmetry in its structure. The optical isomers have identical physical and chemical properties. They only differ in the direction in which they rotate the plane of polarised light.
Geometrical isomerism is not shown by complexes with coordination number 2 and 3, square planar complexes of the type $ M{A_4},M{A_3}B,MA{B_3} $ and octahedral complexes of $ M{A_6},M{A_5}B $ type.
Complete Step By Step Answer:
Geometrical isomerism arises in heteroleptic complexes due to ligands occupying different positions around the central ion. The ligands occupy positions either adjacent to one another or opposite to one another. If the ligands occupy adjacent positions they are referred as cis-form and if the ligands occupies opposite positions they are referred as trans-form.
1,2-dichlorocyclobutane shows geometrical isomerism because it forms cis-1,2-dichlorocyclobutane and trans-1,2-dichlorocyclobutane as shown below

Oximes or N-hydroxy alkane mines also shows geometrical isomerism.

syn oxime is the one which has both hydrogen and hydroxyl groups attached to the $ C = N $ side and in anti oxime the hydrogen and hydroxyl group attached to the opposite of the $ C = N $ $ C = N $ side.
As the attached groups are present in different planes so 1,3-dichloroallene does not show geometrical isomerism.
So the correct answer for the question is option D. 1,2 dichlorocyclobutane and oxime will form geometrical isomers.
Note :
The mirror images compounds are non-superimposable on each other and do not possess the plane of symmetry. The optical isomers also possess the property of chirality.
The essential condition for a substance to show optical activity is that the substance should not have a plane of symmetry in its structure. The optical isomers have identical physical and chemical properties. They only differ in the direction in which they rotate the plane of polarised light.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




