
While writing the following electronic configuration of Fe atom in the ground state some rules have been violated.
Which of the following has/have been violated?
I. Aufbau’s rule
II. Hund’s rule
III. Pauli’s exclusion principle
A. I, II
B. III only
C. I, III
D. I, II, III

Answer
545.4k+ views
Hint: The given three principles Aufbau’s principle, Hund’s rule and Pauli’s exclusion principle tells about the filling of electrons in the atomic orbitals. According to Pauli’s exclusion principle, no two electrons can be present in the orbital with the same spin.
Complete step by step answer:
The Aufbau’s principle tells about the way of filling the electrons in the atomic orbital. The Aufbau’s principle states that electrons are filled in the atomic orbital in the increasing order of the orbital energy. In filling of electrons in the atomic orbital, first electrons are filled in the orbitals with lower energy level than the orbital of higher energy level.
Hund’s rule states that during filling of electrons in the atomic orbital, first each orbital is singly occupied and then doubling of electrons is done in the orbital. The single electrons present in the orbital should have the same spin.
Pauli’s exclusion principle states that in a single atom no two electrons will possess the same set of quantum numbers. Only two electrons can be present in the same orbital and both the electrons should have opposite spin.

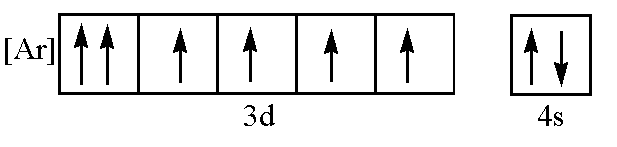
From the given molecular orbital diagram, it can be seen that the 3d orbitals are not filled completely and the 4s orbital is filled completely. So Aufbau’s principle and Hund’s rule both are violated here. In 3d subshell, the first orbital contains two electrons of the same spin, so Pauli’s exclusion principle is violated here.
So, the correct answer is Option D.
Note: In Aufbau's principle, the energy of the atomic orbital depends on the principle quantum number where n = 1, 2, 3……(n-1).
Complete step by step answer:
The Aufbau’s principle tells about the way of filling the electrons in the atomic orbital. The Aufbau’s principle states that electrons are filled in the atomic orbital in the increasing order of the orbital energy. In filling of electrons in the atomic orbital, first electrons are filled in the orbitals with lower energy level than the orbital of higher energy level.
Hund’s rule states that during filling of electrons in the atomic orbital, first each orbital is singly occupied and then doubling of electrons is done in the orbital. The single electrons present in the orbital should have the same spin.
Pauli’s exclusion principle states that in a single atom no two electrons will possess the same set of quantum numbers. Only two electrons can be present in the same orbital and both the electrons should have opposite spin.

From the given molecular orbital diagram, it can be seen that the 3d orbitals are not filled completely and the 4s orbital is filled completely. So Aufbau’s principle and Hund’s rule both are violated here. In 3d subshell, the first orbital contains two electrons of the same spin, so Pauli’s exclusion principle is violated here.
So, the correct answer is Option D.
Note: In Aufbau's principle, the energy of the atomic orbital depends on the principle quantum number where n = 1, 2, 3……(n-1).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




