
Why is beta decay continuous?
Answer
487.5k+ views
Hint: We need to know that $\beta $ decay is not a continuous process but the kinetic energy spectrum of the $\beta $ decay emitted electrons is continuous. A $\beta $ decay process is a type of radioactive decay where an electron is emitted from an atomic nucleus along with an electron antineutrino.
Complete answer:
To solve this problem we can write the $\beta $ decay of carbon $14$ as,
${}_6^{14}C \to {}_7^{14}N + {e^ - } + {v_e}$
If we plot a fraction of electrons having a given kinetic energy against that energy.
Here the X axis represents the electronic kinetic energy and Y-axis represents the energy of that kinetic energy.
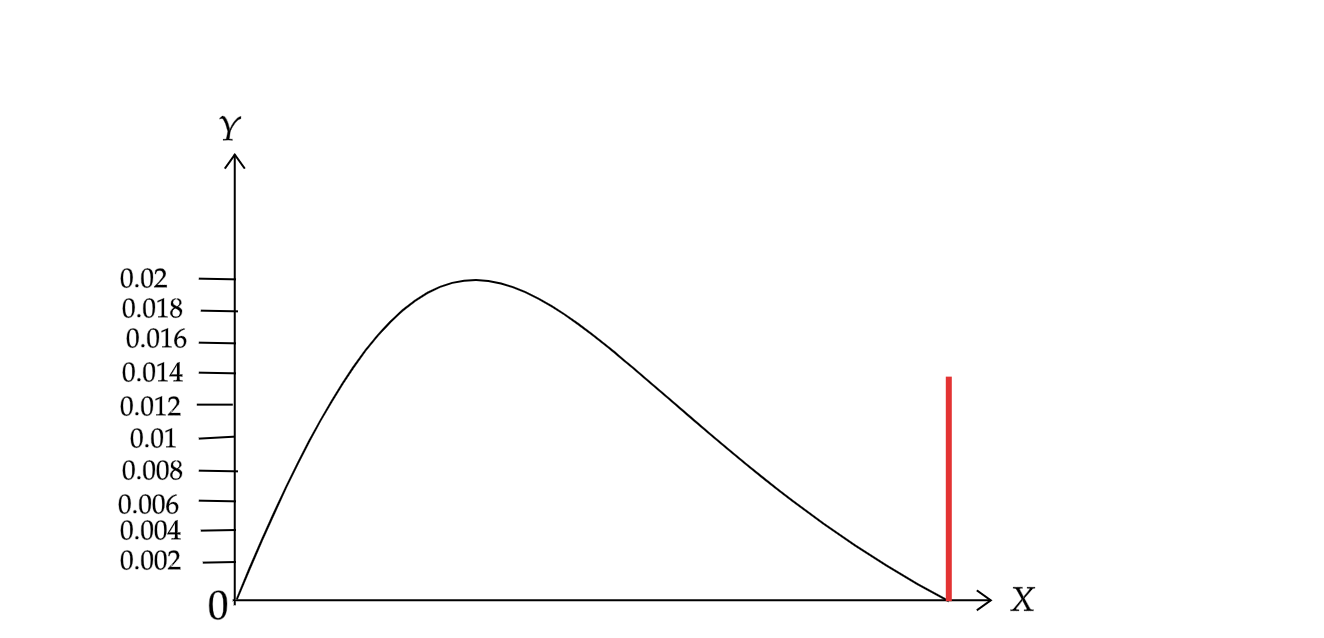
Since from the above equation we can say that the electors are emitted as a stream of discrete particles, $\beta $ decay is not continuous.
Hence we can say that these emitted beta particles have a continuous kinetic energy spectrum. And the energy range is from zero to maximum with available energy is Q. If we carried away only the electrons then the graph would look like the red line as given in the above figure at the right of the graph. Instead we get the continuous energy spectrum in blue. This continuous energy spectrum occurs only when the Q is shared between the electrons and the antineutrino.
Note:
Keep in mind that typical Q is around the kinetic energy of about $1MeV$, but it can range from a few KeV to a few tens of MeV. Remember that the rest mass energy of the electrons is about $511keV$, and most of the energetic $\beta $ particles have a speed close to the speed of light.
Complete answer:
To solve this problem we can write the $\beta $ decay of carbon $14$ as,
${}_6^{14}C \to {}_7^{14}N + {e^ - } + {v_e}$
If we plot a fraction of electrons having a given kinetic energy against that energy.
Here the X axis represents the electronic kinetic energy and Y-axis represents the energy of that kinetic energy.

Since from the above equation we can say that the electors are emitted as a stream of discrete particles, $\beta $ decay is not continuous.
Hence we can say that these emitted beta particles have a continuous kinetic energy spectrum. And the energy range is from zero to maximum with available energy is Q. If we carried away only the electrons then the graph would look like the red line as given in the above figure at the right of the graph. Instead we get the continuous energy spectrum in blue. This continuous energy spectrum occurs only when the Q is shared between the electrons and the antineutrino.
Note:
Keep in mind that typical Q is around the kinetic energy of about $1MeV$, but it can range from a few KeV to a few tens of MeV. Remember that the rest mass energy of the electrons is about $511keV$, and most of the energetic $\beta $ particles have a speed close to the speed of light.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




