
Why is the IR spectrum inverted?
Answer
492.6k+ views
Hint: An Infrared Spectrum (obtained by passing the infrared radiation through the sample of the compound) is the plot of the percentage of transmission of radiation versus the wave number or wavelength of the radiation transmitted.
Complete answer:
Infrared spectroscopy (IR spectroscopy) which uses the infrared region of the electromagnetic spectrum, which is the light with longer wavelength and has lower frequency than visible light. The instrument used for determination of an infrared spectrum is called an IR spectrophotometer. At \[100\% \] transmission all the energy of the particular wavenumber passes through the molecule.
Lower values of percent transmission shows that some of the energy is absorbed by the compound. Each spike which is downward in the IR Spectrum represents absorption of energy. The spikes are called absorption bands. Most of the scientists report the location of absorption bands by using wavenumbers.
Transmittance is the amount of light that successfully passes through the substance without getting reflected or not absorbed and the term Absorbance is the opposite of transmittance which means the amount of light absorbed by the sample.
In the IR spectrum we see that the spectra are plotted upside down because it records the amount of light reaching the detector, i.e. transmission in place of the absorbance. In other words, the absorption peak points downward as it is inverted by the transmittance of the radiation through the sample. This would be clearer by the following graphs:
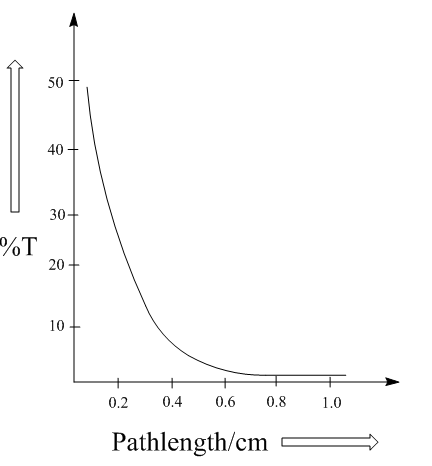
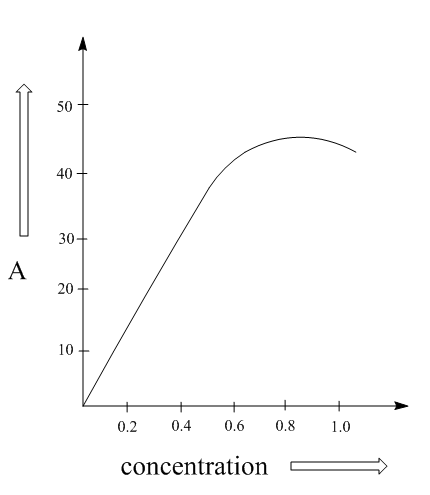
Note:
IR gave information about the presence or absence of specific functional groups. IR can provide a molecular fingerprint that is used to compare samples. If two pure samples show the same IR spectrum then it can have the same compound.
Complete answer:
Infrared spectroscopy (IR spectroscopy) which uses the infrared region of the electromagnetic spectrum, which is the light with longer wavelength and has lower frequency than visible light. The instrument used for determination of an infrared spectrum is called an IR spectrophotometer. At \[100\% \] transmission all the energy of the particular wavenumber passes through the molecule.
Lower values of percent transmission shows that some of the energy is absorbed by the compound. Each spike which is downward in the IR Spectrum represents absorption of energy. The spikes are called absorption bands. Most of the scientists report the location of absorption bands by using wavenumbers.
Transmittance is the amount of light that successfully passes through the substance without getting reflected or not absorbed and the term Absorbance is the opposite of transmittance which means the amount of light absorbed by the sample.
In the IR spectrum we see that the spectra are plotted upside down because it records the amount of light reaching the detector, i.e. transmission in place of the absorbance. In other words, the absorption peak points downward as it is inverted by the transmittance of the radiation through the sample. This would be clearer by the following graphs:


Note:
IR gave information about the presence or absence of specific functional groups. IR can provide a molecular fingerprint that is used to compare samples. If two pure samples show the same IR spectrum then it can have the same compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




