
Why is the pH scale $0 - 14?$
Answer
487.5k+ views
Hint: In this question we have to tell the reason why the pH ranges from $0$ to $14$ . So we will first try to understand the definition of pH and what is the use of it. Further we will also try to understand this from the pH scale. . We know that the degree of alkalinity or acidity of a solution is known as its pH . “pH” is the negative algorithm of hydrogen ion concentration.
Complete answer:
We know that in chemistry, pH is referred to as the potential of hydrogen. pH is a standard scale that is used to measure how acidic or basic a solution is.
We know that pH is the negative logarithm of the hydrogen ion concentration, so we can write the equation to calculate pH as follows:
$pH = - \log \left[ {{H^ + }} \right]$
The units of pH are moles per litre.
We know that the acids have pH range from $0$ to $7$. The point $7$ is neutral and the basic solutions have pH above $7$ . It can go up to $14$. And we know that the most acidic solutions have a smaller range i.e. near $2$, but the most basic solutions have a pH range of higher value near $12$.
We can see this from the diagram of the pH scale:
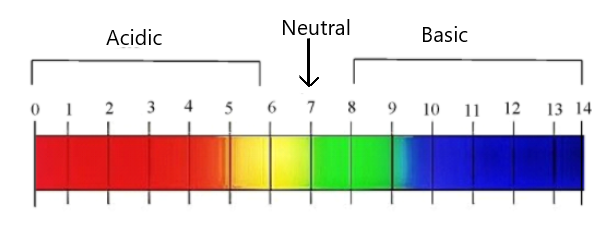
Hence the pH value ranges from $0 - 14$ .
Note:
We should know that a solution that has more ${H^ + }$ in it will have more pH . It must be noted that the pH of pure water is neutral. The pH of blood is light. We can use the pH meter to measure the pH of the solution. We should know that the scientist who developed the pH scale is Sorenson.
Complete answer:
We know that in chemistry, pH is referred to as the potential of hydrogen. pH is a standard scale that is used to measure how acidic or basic a solution is.
We know that pH is the negative logarithm of the hydrogen ion concentration, so we can write the equation to calculate pH as follows:
$pH = - \log \left[ {{H^ + }} \right]$
The units of pH are moles per litre.
We know that the acids have pH range from $0$ to $7$. The point $7$ is neutral and the basic solutions have pH above $7$ . It can go up to $14$. And we know that the most acidic solutions have a smaller range i.e. near $2$, but the most basic solutions have a pH range of higher value near $12$.
We can see this from the diagram of the pH scale:

Hence the pH value ranges from $0 - 14$ .
Note:
We should know that a solution that has more ${H^ + }$ in it will have more pH . It must be noted that the pH of pure water is neutral. The pH of blood is light. We can use the pH meter to measure the pH of the solution. We should know that the scientist who developed the pH scale is Sorenson.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




