
Write a reaction which shows that all the atoms in glucose are linked in a straight chain.
Answer
581.4k+ views
Hint: Glucose is a carbohydrate that contains carbon, hydrogen and oxygen. It is a polyhydroxy aldehyde.
Complete step by step answer:
Glucose contains one primary alcoholic group, four secondary alcoholic groups and one aldehyde group. The structure of glucose is as follows:
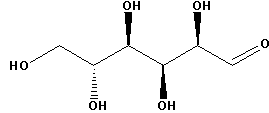
To show that all the atoms in glucose are linked in a straight chain, many reactions with different reagents can be carried out. We can then analyse the products of all the reactions and the structures of the products can be related with the structure of reactant i.e. glucose.
The reaction of glucose with hydroiodic acid is as follows:
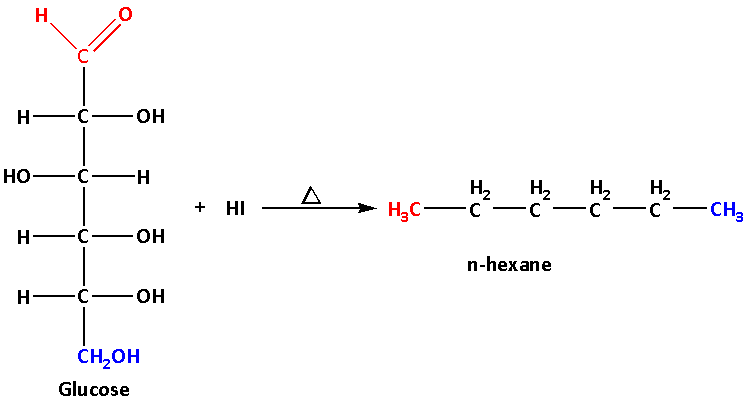
The primary alcoholic group is shown in blue colour, the secondary alcoholic groups are shown in black colour and the aldehyde group is shown in red colour. These groups after reduction with hydroiodic acid are shown in their respective colours.
The hydroiodic acid is a reducing agent. It reduces primary alcohols, secondary alcohols and aldehyde groups.
Glucose on heating with hydroiodic acid gives n-hexane. The hydroiodic acid reduces the one primary alcoholic group of glucose to one methyl group, the four secondary alcoholic groups to four methylene groups and the one aldehyde group to one methyl group.
Glucose on reduction with hydroiodic acid gives n-hexane which a straight chain alkane. The straight chain alkane contains six carbon atoms linked in a straight chain. During the reaction no carbon-carbon bond is broken. Thus suggests that the structure of glucose contains six carbon atoms linked in a straight chain.
Note: Hydroiodic acid is a reducing agent. Thus, do not write the oxidation product such as hexanoic acid. On prolonged heating with HI glucose will give n- hexane.
Complete step by step answer:
Glucose contains one primary alcoholic group, four secondary alcoholic groups and one aldehyde group. The structure of glucose is as follows:

To show that all the atoms in glucose are linked in a straight chain, many reactions with different reagents can be carried out. We can then analyse the products of all the reactions and the structures of the products can be related with the structure of reactant i.e. glucose.
The reaction of glucose with hydroiodic acid is as follows:

The primary alcoholic group is shown in blue colour, the secondary alcoholic groups are shown in black colour and the aldehyde group is shown in red colour. These groups after reduction with hydroiodic acid are shown in their respective colours.
The hydroiodic acid is a reducing agent. It reduces primary alcohols, secondary alcohols and aldehyde groups.
Glucose on heating with hydroiodic acid gives n-hexane. The hydroiodic acid reduces the one primary alcoholic group of glucose to one methyl group, the four secondary alcoholic groups to four methylene groups and the one aldehyde group to one methyl group.
Glucose on reduction with hydroiodic acid gives n-hexane which a straight chain alkane. The straight chain alkane contains six carbon atoms linked in a straight chain. During the reaction no carbon-carbon bond is broken. Thus suggests that the structure of glucose contains six carbon atoms linked in a straight chain.
Note: Hydroiodic acid is a reducing agent. Thus, do not write the oxidation product such as hexanoic acid. On prolonged heating with HI glucose will give n- hexane.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




