
Write a short note on osmosis.
Answer
521.1k+ views
Hint: Osmosis is the movement of solvent molecules across a semipermeable membrane toward a region of higher concentration of solute molecules. The main dissimilarity between osmosis and the process called diffusion is that the latter does not require a semipermeable membrane. Osmosis is a biophysical phenomenon occurring in all biological systems.
Complete answer:
In biotic systems, the water molecules pass through the cell membrane from an area of low concentration of solute to a region of high concentration of solute so as to enable the entry and exit of important nutrients for the cell. For example, if a particular cell is submerged in saltwater, water molecules will move out of the cell. If that cell is submerged in freshwater, water molecules will move into the cell. The turgor pressure of a cell is maintained by osmosis between the cell interior and the cell’s hypotonic environment. When the cell membrane has a volume of pure water on both its sides, water molecules will pass in and out in each direction at exactly the same rate. Thus, there will be no net flow of water through the cell membrane. An animal cell has thin walls whereas a plant cell has thick walls. Due to the plant’s thick walls, the cells require more water. These cells won’t burst in a hypotonic solution. In fact, a hypotonic solution is the ideal solution for a plant cell. On the contrary, an animal cell will survive only in an isotonic solution. The leaves of a plant will droop when kept in an isotonic solution. Osmosis is the process by means of which cells maintain their water content and its turgidity.
Note:
In biological systems, the solvent is usually water, but osmosis can occur in other liquids, supercritical liquids and even gases. Turgor pressure is the force within the cell that pushes the plasma membrane (the cell membrane) against the cell wall. Isotonic solution and hypotonic solution are two of the types of osmotic solutions, the other one being hypertonic solution. Isotonic indicates the same concentration of solution inside and outside the cell whereas hypertonic indicates higher concentration of solution outside the cell. Hypotonic solution is the one that has higher concentration of solution inside the cell.
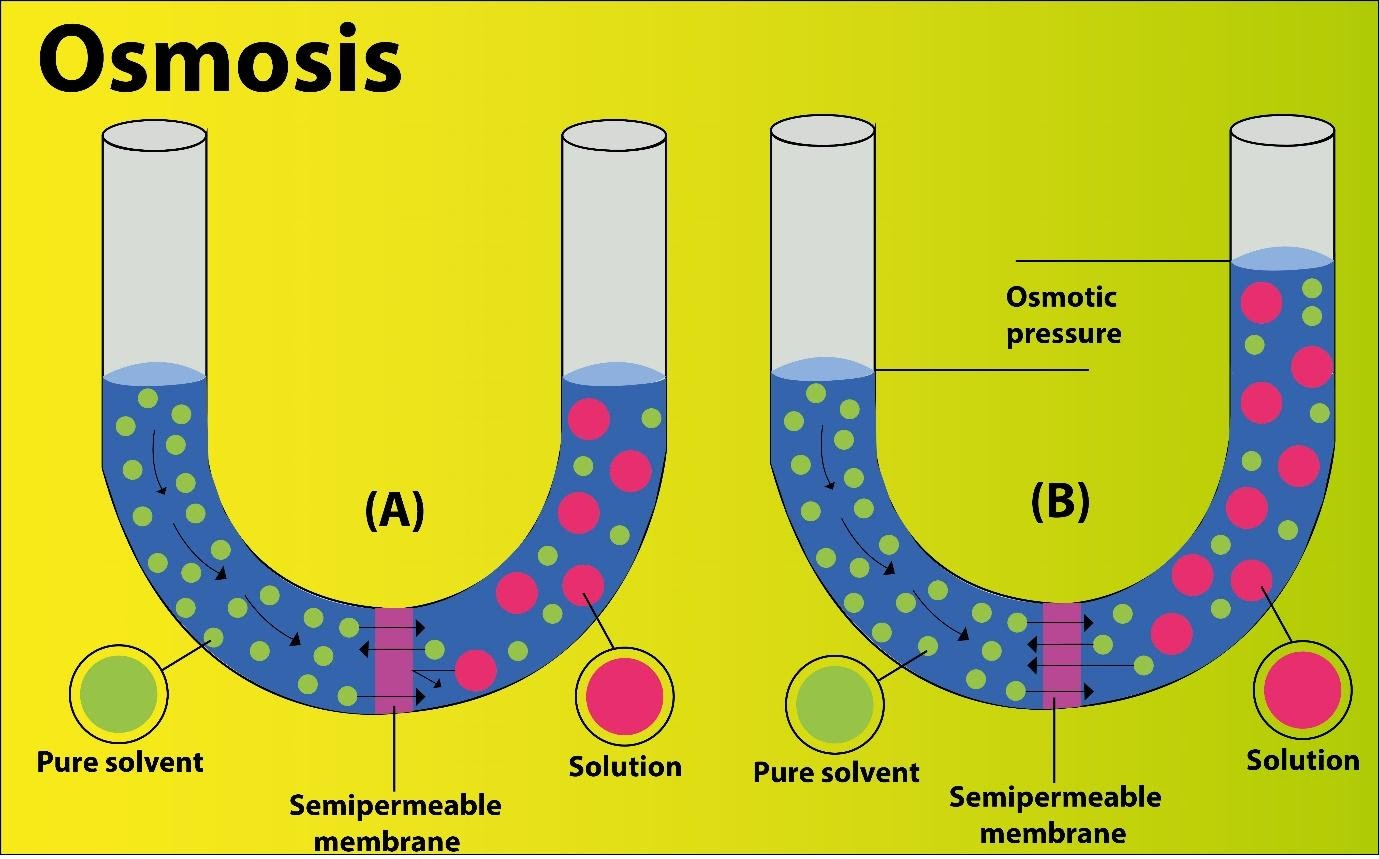
Figure: Osmosis
Complete answer:
In biotic systems, the water molecules pass through the cell membrane from an area of low concentration of solute to a region of high concentration of solute so as to enable the entry and exit of important nutrients for the cell. For example, if a particular cell is submerged in saltwater, water molecules will move out of the cell. If that cell is submerged in freshwater, water molecules will move into the cell. The turgor pressure of a cell is maintained by osmosis between the cell interior and the cell’s hypotonic environment. When the cell membrane has a volume of pure water on both its sides, water molecules will pass in and out in each direction at exactly the same rate. Thus, there will be no net flow of water through the cell membrane. An animal cell has thin walls whereas a plant cell has thick walls. Due to the plant’s thick walls, the cells require more water. These cells won’t burst in a hypotonic solution. In fact, a hypotonic solution is the ideal solution for a plant cell. On the contrary, an animal cell will survive only in an isotonic solution. The leaves of a plant will droop when kept in an isotonic solution. Osmosis is the process by means of which cells maintain their water content and its turgidity.
Note:
In biological systems, the solvent is usually water, but osmosis can occur in other liquids, supercritical liquids and even gases. Turgor pressure is the force within the cell that pushes the plasma membrane (the cell membrane) against the cell wall. Isotonic solution and hypotonic solution are two of the types of osmotic solutions, the other one being hypertonic solution. Isotonic indicates the same concentration of solution inside and outside the cell whereas hypertonic indicates higher concentration of solution outside the cell. Hypotonic solution is the one that has higher concentration of solution inside the cell.

Figure: Osmosis
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




