
Write Bohr’s postulates for the hydrogen atom model.
Answer
610.5k+ views
Hint:In order to solve this question, we must know the concept of Bohr’s model of the hydrogen atom. Along with that, we must know the three postulates of Bohr’s model of the hydrogen atom.
Complete step by step answer:
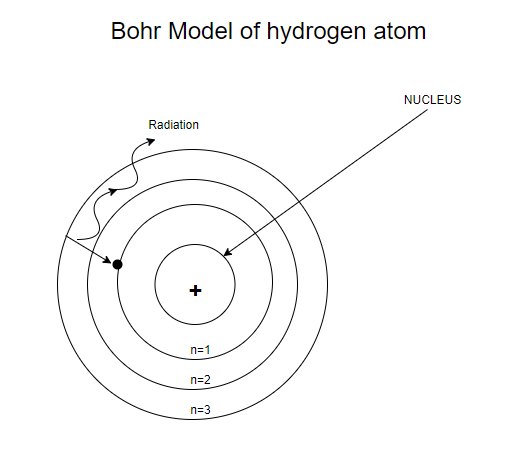
Bohr’s model of the hydrogen atom-
It explains the emission and absorption spectra of atomic hydrogen and hydrogen-like ions with low atomic numbers. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Bohr’s model is an important step in the development of quantum mechanics, which deals with many-electron atoms.
Bohr’s model of the hydrogen atom is based on three postulates-
First postulate- Electron revolves around the nucleus in discrete circular orbits called stationary orbits without emission of radiant energy. These orbits are called stable orbits or non-radiating orbits.
Second postulate- Electrons revolve around the nucleus only in orbits in which their angular momentum is an integral multiple of $\dfrac{h}{{2\pi }}$.
Third postulate- When an electron makes a transition from one of its non-radiating orbits to another of lower energy, a photon is emitted having energy equal to the energy difference between the two states. The frequency of the emitted photon is then given by, $v = \dfrac{{{E_i} - {E_f}}}{h}$
Note- We should know that Bohr’s model is semi-classical because it combines the classical concept of electron orbit i.e. postulate 1 with the new concept of quantization i.e. postulate 2 and postulate 3. Also we should know that it is an important step in the development of quantum mechanics.
Complete step by step answer:

Bohr’s model of the hydrogen atom-
It explains the emission and absorption spectra of atomic hydrogen and hydrogen-like ions with low atomic numbers. It was the first model to introduce the concept of a quantum number to describe atomic states and to postulate quantization of electron orbits in the atom. Bohr’s model is an important step in the development of quantum mechanics, which deals with many-electron atoms.
Bohr’s model of the hydrogen atom is based on three postulates-
First postulate- Electron revolves around the nucleus in discrete circular orbits called stationary orbits without emission of radiant energy. These orbits are called stable orbits or non-radiating orbits.
Second postulate- Electrons revolve around the nucleus only in orbits in which their angular momentum is an integral multiple of $\dfrac{h}{{2\pi }}$.
Third postulate- When an electron makes a transition from one of its non-radiating orbits to another of lower energy, a photon is emitted having energy equal to the energy difference between the two states. The frequency of the emitted photon is then given by, $v = \dfrac{{{E_i} - {E_f}}}{h}$
Note- We should know that Bohr’s model is semi-classical because it combines the classical concept of electron orbit i.e. postulate 1 with the new concept of quantization i.e. postulate 2 and postulate 3. Also we should know that it is an important step in the development of quantum mechanics.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




