
Write chemical reaction to affect following transformation: 3-Nitrobromobenzene to 3-Nitrobenzoic acid.
Answer
565.2k+ views
Hint: 3- nitro bromobenzene can be converted into 3-Nitrobenzoic acid by 2 methods.
In the first method, 3-Nitrobromobenzene is reacted with \[Mg\] metal followed by hydrolysis. In the second method, firstly \[ethanolic{{ }}KCN\] is used followed by acidic hydrolysis.
Complete step by step answer:
Before heading on to the conversion, we need to know a little about 3-Nitrobromobenzene. So, this is an organic compound with molecular formula as: \[{C_6}{H_4}BrN{O_2}\]. It is a white to yellow colored crystal powder used in labeled glycerol kinase substrate specificity.
If we replace the Bromo group in 3-Nitrobromobenzene with \[COOH\] group, we will get our desired product i.e., 3-Nitrobenzoic acid. This can be done by 2 methods. We will discuss each method in brief.
First method: In this method, we will react to our reactant \[Mg\] metal dissolved in ether. Here, we used ether as our solvent because it is aprotic and will solvate the magnesium ion completely. A Grignard reagent will form as a product as the \[Mg\] will attach to the halide present in the reactant. After that, we will take carbon dioxide in the form of dry ice, this will react the Grignard reagent followed by hydrolysis and we will get the final product as 3-Nitrobenzoic acid as shown below:
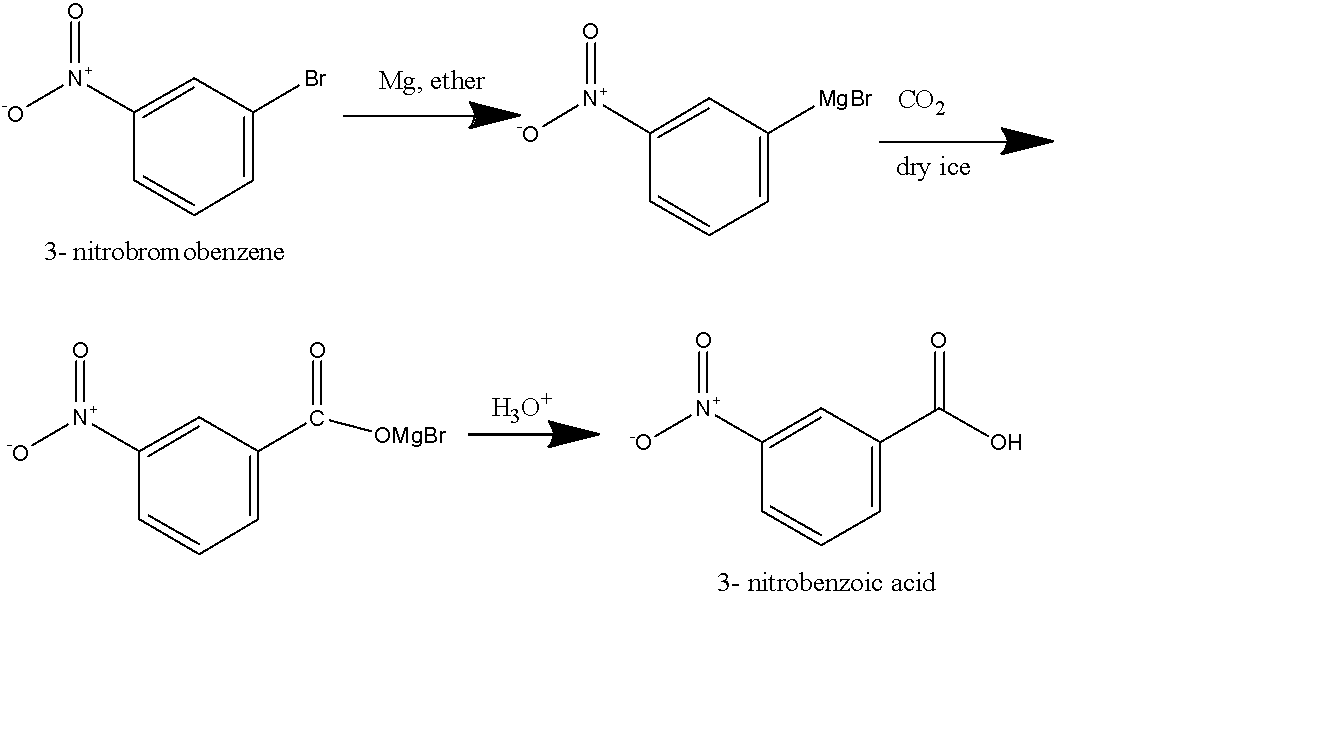
Second method: in this method, we use \[KCN\] dissolved in an alcoholic solvent, it will generate the nitrile group on the benzene ring replacing the Bromo group, and will form 3- Nitro benzonitrile as shown in the figure below. 3- Nitro benzonitrile will undergo acidic hydrolysis to give 3-Nitrobenzoic acid as the final product as shown below:
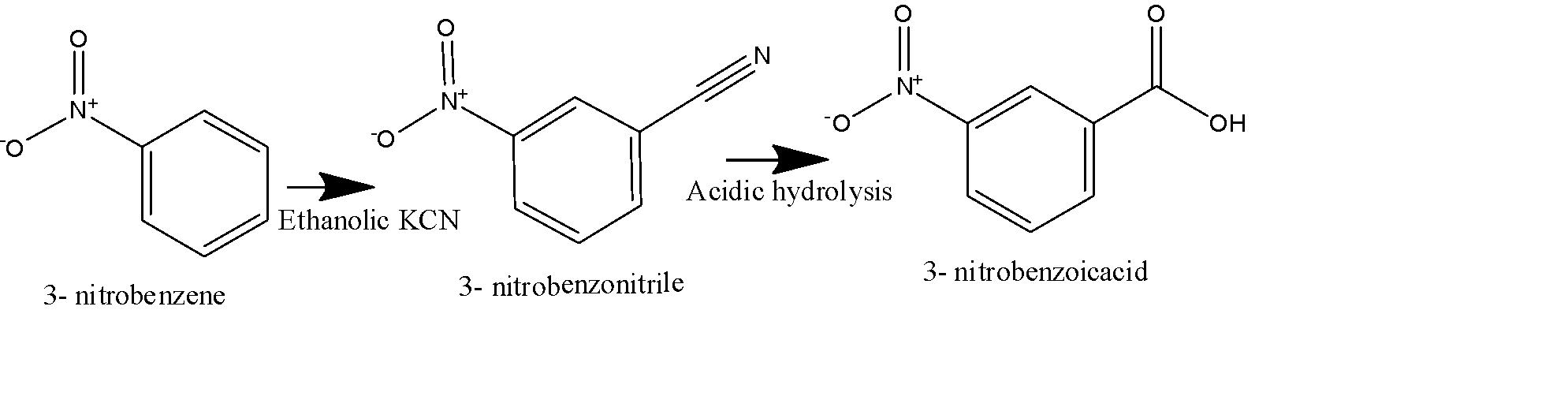
Note: In the first reaction, carbon dioxide provides the additional carbon for the acidic \[COOH\] group, and in the second reaction nitrile group provides that additional carbon.
Acidic hydrolysis is a type of nucleophilic reaction involving the cleavage of the bond by using a protic acid as a catalyst.
In the first method, 3-Nitrobromobenzene is reacted with \[Mg\] metal followed by hydrolysis. In the second method, firstly \[ethanolic{{ }}KCN\] is used followed by acidic hydrolysis.
Complete step by step answer:
Before heading on to the conversion, we need to know a little about 3-Nitrobromobenzene. So, this is an organic compound with molecular formula as: \[{C_6}{H_4}BrN{O_2}\]. It is a white to yellow colored crystal powder used in labeled glycerol kinase substrate specificity.
If we replace the Bromo group in 3-Nitrobromobenzene with \[COOH\] group, we will get our desired product i.e., 3-Nitrobenzoic acid. This can be done by 2 methods. We will discuss each method in brief.
First method: In this method, we will react to our reactant \[Mg\] metal dissolved in ether. Here, we used ether as our solvent because it is aprotic and will solvate the magnesium ion completely. A Grignard reagent will form as a product as the \[Mg\] will attach to the halide present in the reactant. After that, we will take carbon dioxide in the form of dry ice, this will react the Grignard reagent followed by hydrolysis and we will get the final product as 3-Nitrobenzoic acid as shown below:

Second method: in this method, we use \[KCN\] dissolved in an alcoholic solvent, it will generate the nitrile group on the benzene ring replacing the Bromo group, and will form 3- Nitro benzonitrile as shown in the figure below. 3- Nitro benzonitrile will undergo acidic hydrolysis to give 3-Nitrobenzoic acid as the final product as shown below:

Note: In the first reaction, carbon dioxide provides the additional carbon for the acidic \[COOH\] group, and in the second reaction nitrile group provides that additional carbon.
Acidic hydrolysis is a type of nucleophilic reaction involving the cleavage of the bond by using a protic acid as a catalyst.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




