
Write dash formulae for the following bond line formula.
(A)
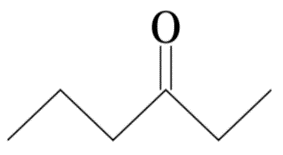
(B)
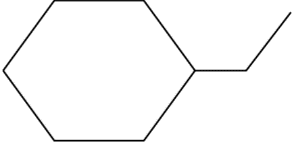
(C)
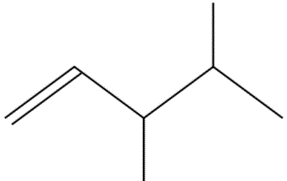
(D)




Answer
510.3k+ views
Hint: First we have to know that bond line structure is a structure representation of organic compounds where carbon and hydrogen atoms are not indicated, but only the bonds between carbons atoms are indicated in the form of lines. The chemical formula in which there is no presence of sigma or pi bonds is called condensed formula. In the given compounds, there are carbons that have methyl and ethyl groups attached to the sides and these contribute to increasing the molar mass of the compound.
Complete answer:
Dash Formula is a representation of all the bonds of the compound using dash that specifies the bond between the two atoms. We know that carbon has four valencies. That means one carbon can attach with itself four other groups or atoms, so that its octet gets filled. Also single line ( $ - $ ) represents a single $ C $ to $ C $ bond, a double line ( $ = $ ) indicates a double $ C $ to $ C $ bond and a $ C $ to $ C $ triple bond is shown by a triple line ( $ \equiv $ ).
a.
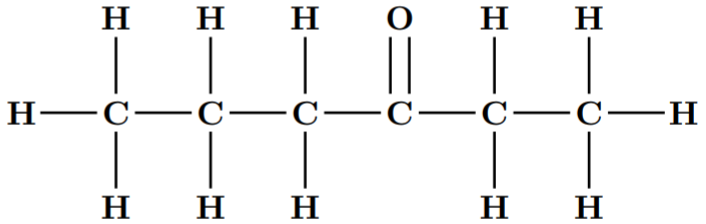
The given compound is 3-Hexanone or Ethyl propyl ketone. Its molecular formula is $ {C_6}{H_{12}}O $ .
The dash formula of this compound is:
b.
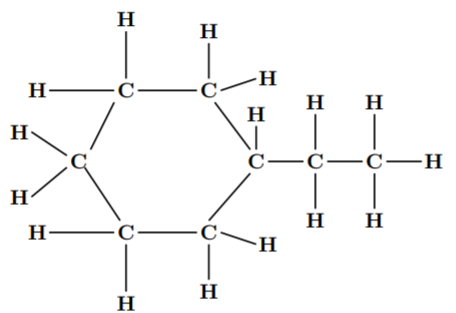
The given compound is 1- ethyl-hexane. Its chemical formula is $ {C_8}{H_{16}} $ .
The dash formula of this compound is:
c.
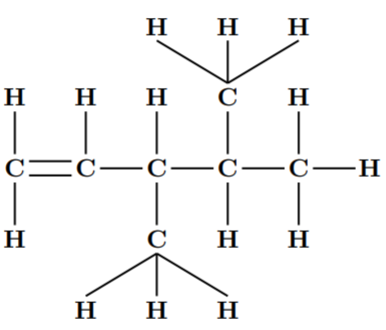
The given compound is 3,4-dimethylpent-1-ene. Its molecular formula is $ {C_6}{H_{14}} $ .
The Dash formula for this compound is:
d.
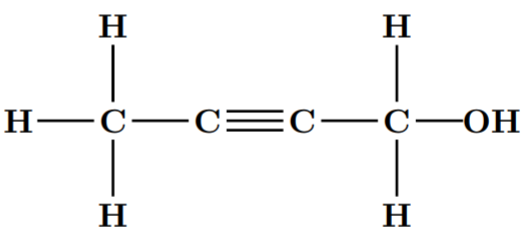
Note:
Note that the organic compound can be depicted in many ways like complete structure which is also known as dash formula, condensed structure which we say as molecular formula and bond line structure. The bond line formula is for drawing two-dimensional organic structures. Also note that $ n $ dash denoted $ 2n $ electrons are shared between two atoms.
Complete answer:
Dash Formula is a representation of all the bonds of the compound using dash that specifies the bond between the two atoms. We know that carbon has four valencies. That means one carbon can attach with itself four other groups or atoms, so that its octet gets filled. Also single line ( $ - $ ) represents a single $ C $ to $ C $ bond, a double line ( $ = $ ) indicates a double $ C $ to $ C $ bond and a $ C $ to $ C $ triple bond is shown by a triple line ( $ \equiv $ ).
a.

The given compound is 3-Hexanone or Ethyl propyl ketone. Its molecular formula is $ {C_6}{H_{12}}O $ .
The dash formula of this compound is:
b.

The given compound is 1- ethyl-hexane. Its chemical formula is $ {C_8}{H_{16}} $ .
The dash formula of this compound is:
c.

The given compound is 3,4-dimethylpent-1-ene. Its molecular formula is $ {C_6}{H_{14}} $ .
The Dash formula for this compound is:
d.
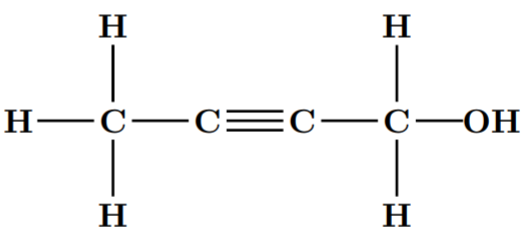
Note:
Note that the organic compound can be depicted in many ways like complete structure which is also known as dash formula, condensed structure which we say as molecular formula and bond line structure. The bond line formula is for drawing two-dimensional organic structures. Also note that $ n $ dash denoted $ 2n $ electrons are shared between two atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




