
Write down the formulae of aluminium chloride.
Answer
590.4k+ views
Hint: By finding the charge on aluminium and chloride and then making the valence criss-cross structure of it we will get our formula. Aluminium chloride has one Al atom and three Cl atoms, it is as a Ionic compound as the Al electron is passed to three atoms of Cl.
Complete step by step answer:
First let’s see what are the symbols for the Aluminium and Chloride which are $Al$ for Aluminium and $Cl$ for Chloride.
Now coming to the term ions where it is of two types positive and negative known as cation and anion.
Here Aluminium has$3$ positive charge because aluminium has three valence electron so it tend to lose electrons then after losing these $3$ electrons it has $10$ electrons and $13$ protons and these $10$ electrons are neutralized by$10$protons and out of $13$ protons so we get $3$ positive charge because of excess three protons in it and when we talk about Chloride which has $1$ negative charge because here it is more energy-efficient and easy for chlorine to gain$1$ electron than to lose seven electron so it tend to gain an electron to create an ion with $17$ protons, $17$ neutrons and $18$ electrons, which give it a net negative charge of $-1$ which is also referred as the chloride ion.
Now doing valence criss-cross we get it as
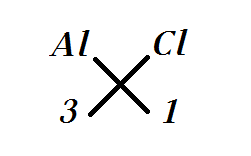
By which we get the formula for Aluminium Chloride as $AlC{{l}_{3}}$.
Note: -The criss-cross method is an alternative way to write the chemical formula in which the numeric value of each of the ion charges is crossed over to make the subscript of the other ion, the important point in it is that the signs of the charge like negative and positive are dropped.
-It is white, but iron trichloride is also polluted by samples, giving it a yellow colour. There is a low melting and boiling point of the solid.
Complete step by step answer:
First let’s see what are the symbols for the Aluminium and Chloride which are $Al$ for Aluminium and $Cl$ for Chloride.
Now coming to the term ions where it is of two types positive and negative known as cation and anion.
Here Aluminium has$3$ positive charge because aluminium has three valence electron so it tend to lose electrons then after losing these $3$ electrons it has $10$ electrons and $13$ protons and these $10$ electrons are neutralized by$10$protons and out of $13$ protons so we get $3$ positive charge because of excess three protons in it and when we talk about Chloride which has $1$ negative charge because here it is more energy-efficient and easy for chlorine to gain$1$ electron than to lose seven electron so it tend to gain an electron to create an ion with $17$ protons, $17$ neutrons and $18$ electrons, which give it a net negative charge of $-1$ which is also referred as the chloride ion.
Now doing valence criss-cross we get it as
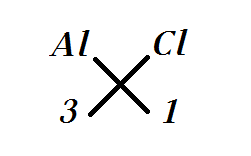
By which we get the formula for Aluminium Chloride as $AlC{{l}_{3}}$.
Note: -The criss-cross method is an alternative way to write the chemical formula in which the numeric value of each of the ion charges is crossed over to make the subscript of the other ion, the important point in it is that the signs of the charge like negative and positive are dropped.
-It is white, but iron trichloride is also polluted by samples, giving it a yellow colour. There is a low melting and boiling point of the solid.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




