
: Write down the formulae of sodium sulfide.
Answer
583.8k+ views
Hint: We know that, joining of atoms of different elements in definite proportions forms compounds. Some examples of compounds are water $\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}} \right)$, ammonia $\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)$. In water, hydrogen and oxygen are present in the fixed ratio of 1:8.
Complete step by step answer:
Let's discuss the writing formula of a compound. Chemical formula of a compound shows the composition of the compound using symbols of combining atoms. To write the formula, we need the valency of the combining elements.
Let’s discuss valency of an element. The capacity of combination of elements is termed as valency. In simple words valency gives the idea how an atom of an element combines with atoms of another element. Valency of sodium is +1, magnesium is +2 and aluminium is +3 etc.
To write the chemical formula of a compound, first we have to write the chemical symbols of the elements along with the valency then, we have to crossover the valencies of the combining elements.
Let’s come to question. We have to write the formula of sodium sulfide. So, the two elements present in the compound are sodium and sulphur. The valency of sodium is +1 and sulphur is -2.
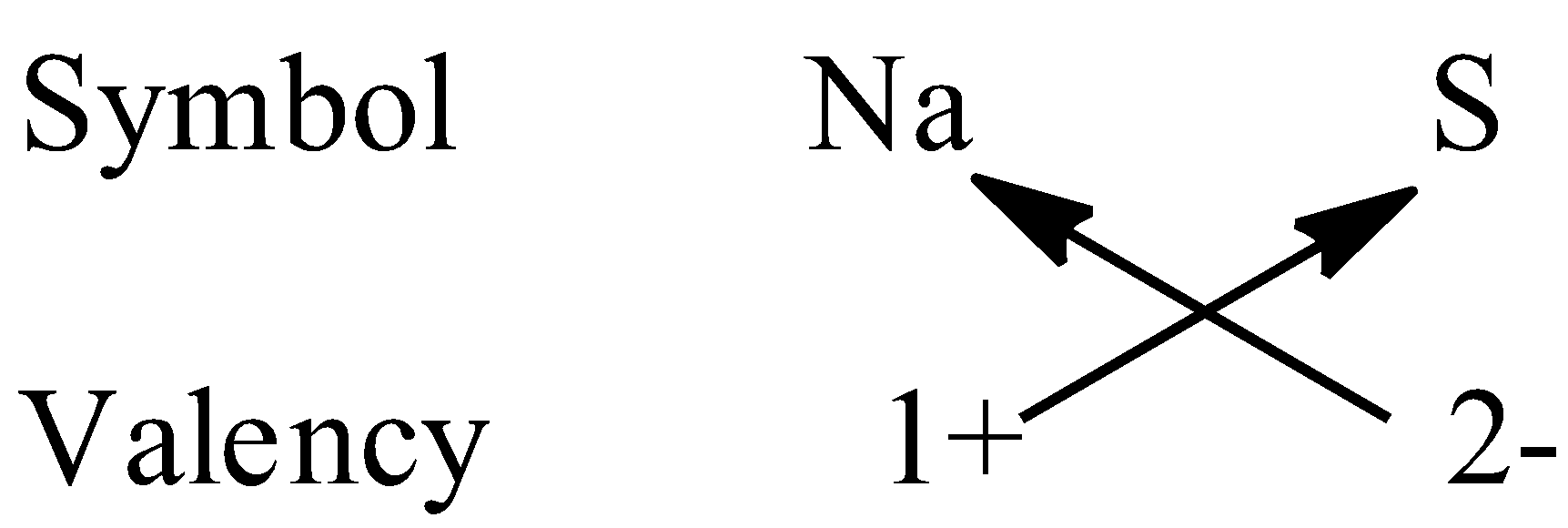
So, the formula of sodium sulphide is ${\rm{N}}{{\rm{a}}_{\rm{2}}}{\rm{S}}$.
Note:
There are some rules to be followed while writing chemical formulas of compounds
1) In compounds having a metal and non-metal, the symbol of metal is to be written first. For example in the compound calcium oxide (CaO), calcium is written first as it is a metal.
2) The valencies on the ion must balance
3) In case of compounds having polyatomic ions such as hydroxide ion (OH), polyatomic ion is to be enclosed within brackets.
Complete step by step answer:
Let's discuss the writing formula of a compound. Chemical formula of a compound shows the composition of the compound using symbols of combining atoms. To write the formula, we need the valency of the combining elements.
Let’s discuss valency of an element. The capacity of combination of elements is termed as valency. In simple words valency gives the idea how an atom of an element combines with atoms of another element. Valency of sodium is +1, magnesium is +2 and aluminium is +3 etc.
To write the chemical formula of a compound, first we have to write the chemical symbols of the elements along with the valency then, we have to crossover the valencies of the combining elements.
Let’s come to question. We have to write the formula of sodium sulfide. So, the two elements present in the compound are sodium and sulphur. The valency of sodium is +1 and sulphur is -2.
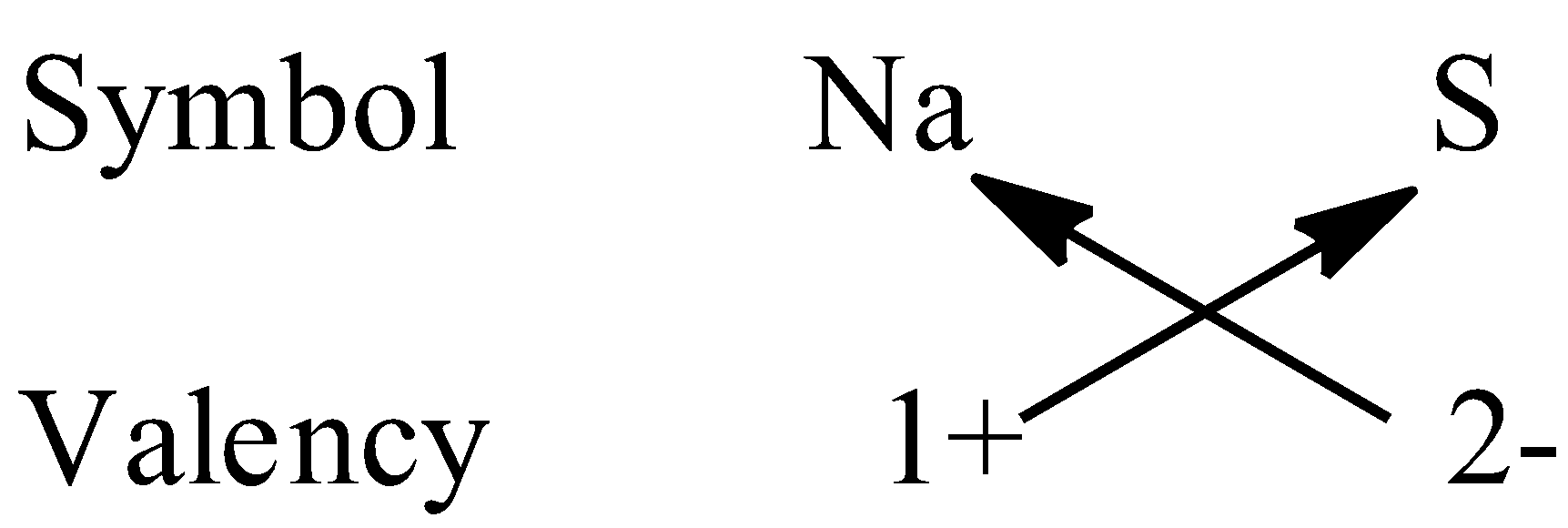
So, the formula of sodium sulphide is ${\rm{N}}{{\rm{a}}_{\rm{2}}}{\rm{S}}$.
Note:
There are some rules to be followed while writing chemical formulas of compounds
1) In compounds having a metal and non-metal, the symbol of metal is to be written first. For example in the compound calcium oxide (CaO), calcium is written first as it is a metal.
2) The valencies on the ion must balance
3) In case of compounds having polyatomic ions such as hydroxide ion (OH), polyatomic ion is to be enclosed within brackets.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




