
Write down the possible isomers and give their IUPAC names using the formula ${C_4}{H_{10}}$ .
Answer
580.5k+ views
Hint: The compounds having the same molecular formula but different structural formulas are called isomers of each other and the phenomenon is called Isomerism.
${C_4}{H_{10}}$ has only two isomers - n-butane and isobutene.
Complete step by step answer:
Butane is an alkane with four carbon atoms. Its molecular formula is ${C_4}{H_{10}}$ . Its two isomers are n-butane and isobutane or 2 - methylpropane.
n-butane: n butane or normal butane or butane is a colourless gas with a faint petroleum like odour. Its average mass is 58.122 Da and monoisotopic mass is 58.07251 Da.
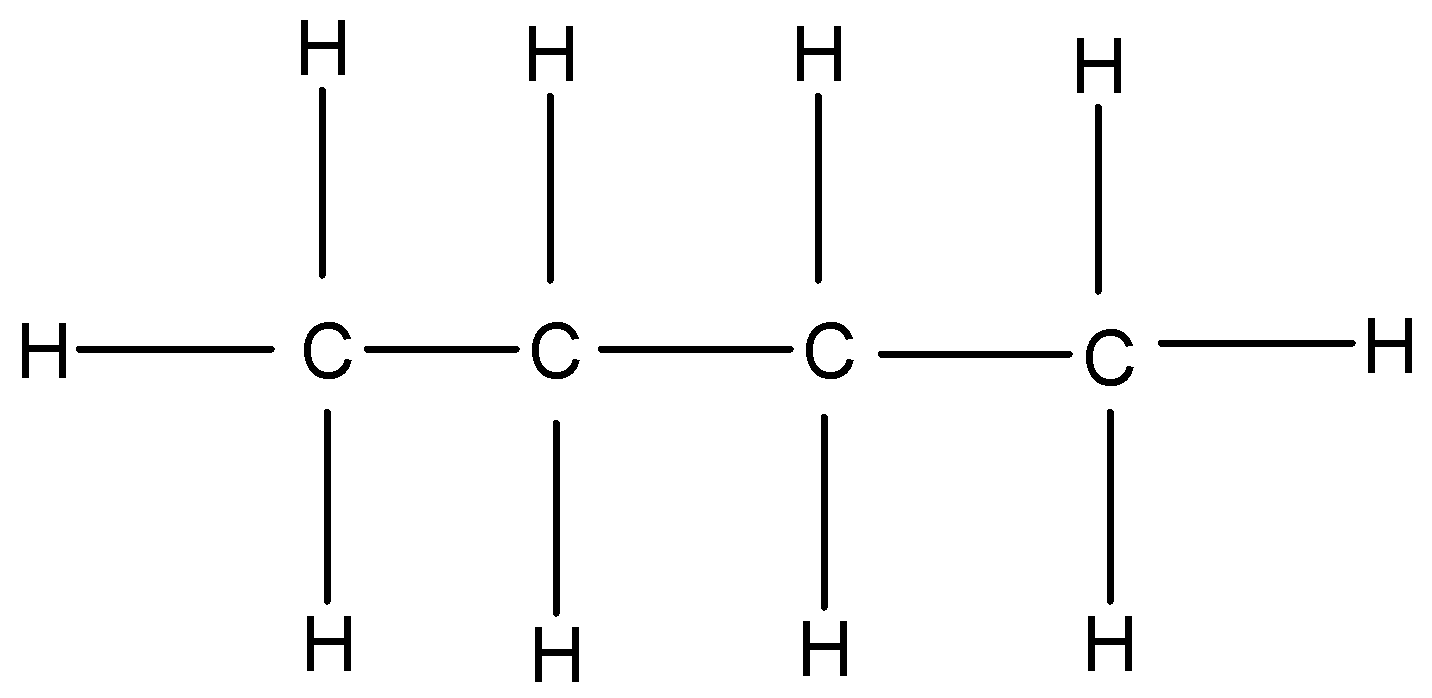
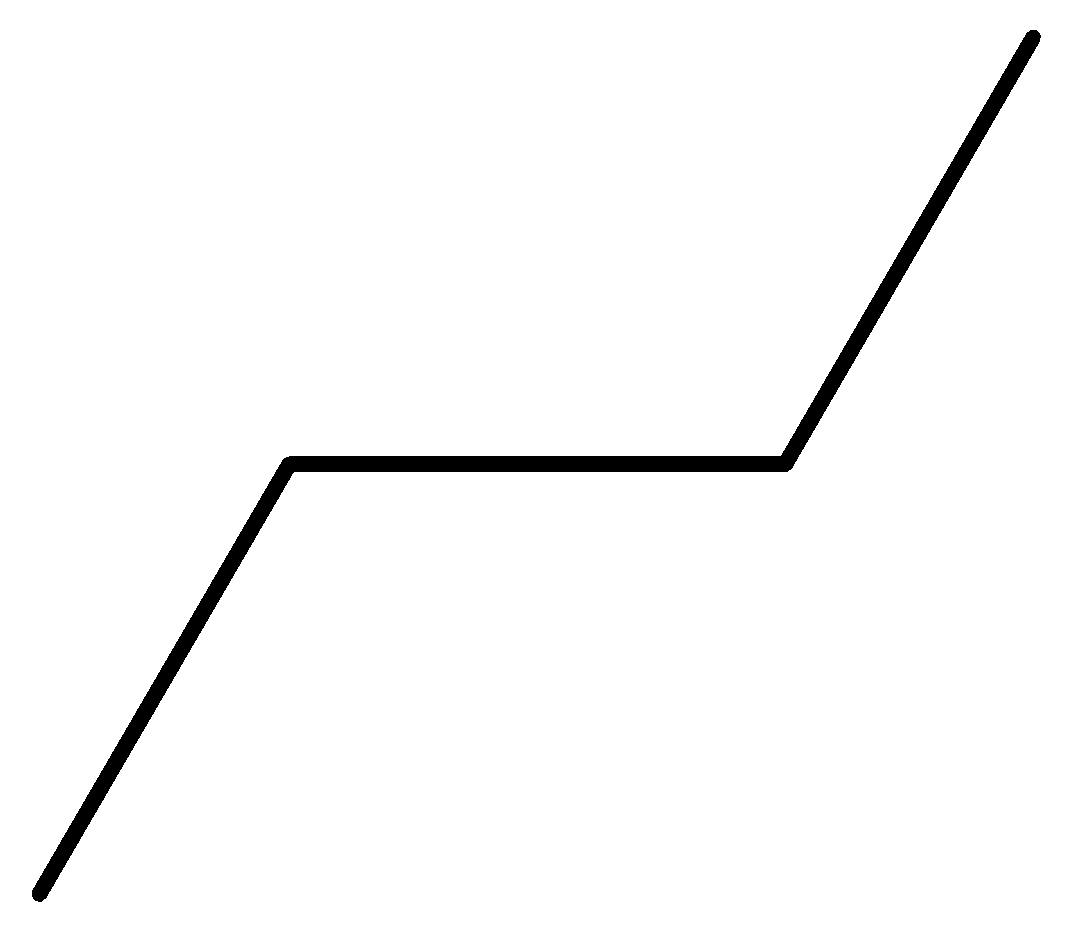
Figure: Structure formula of n-butane
Iso-butane: The other isomer is iso-butene or 2-methyl propane or i-butane. Iso-butane has a propane parent chain with a methyl group \[-CH3\] attached to the second carbon of the chain. That is why its IUPAC name is 2-methyl propane.
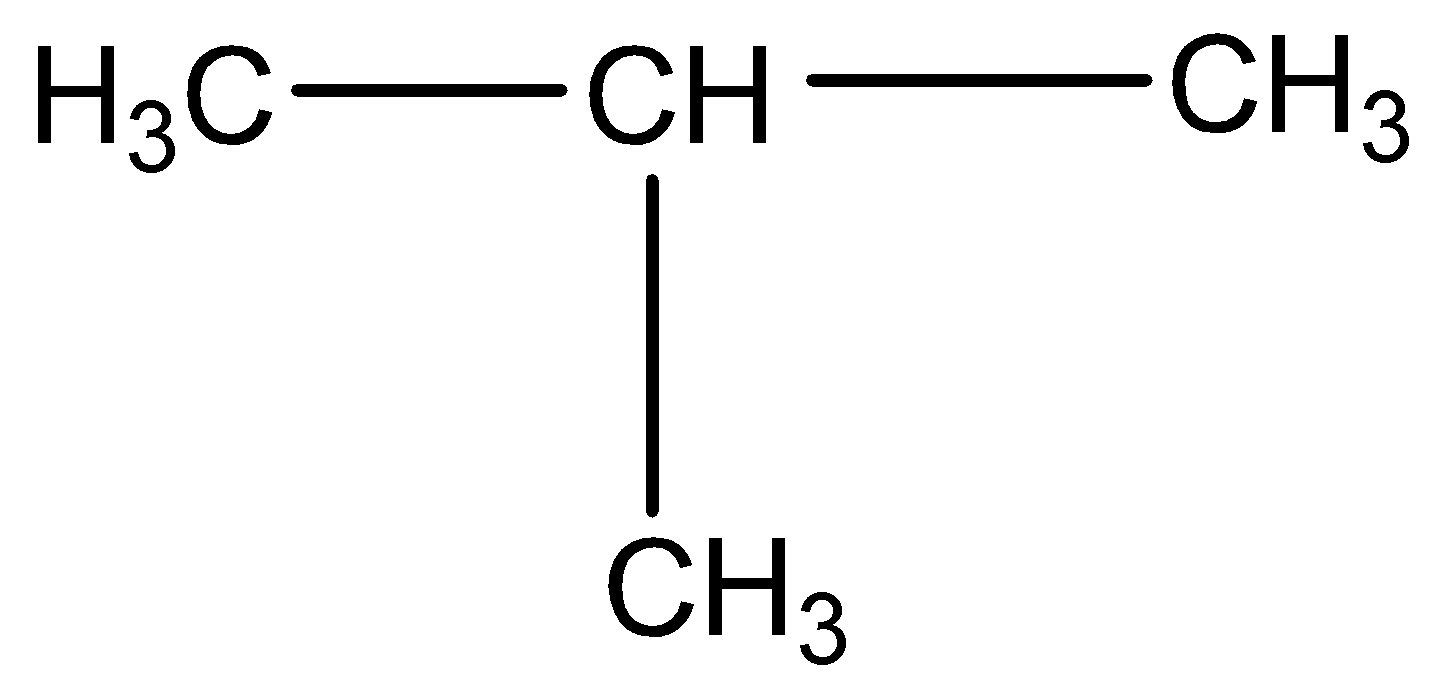
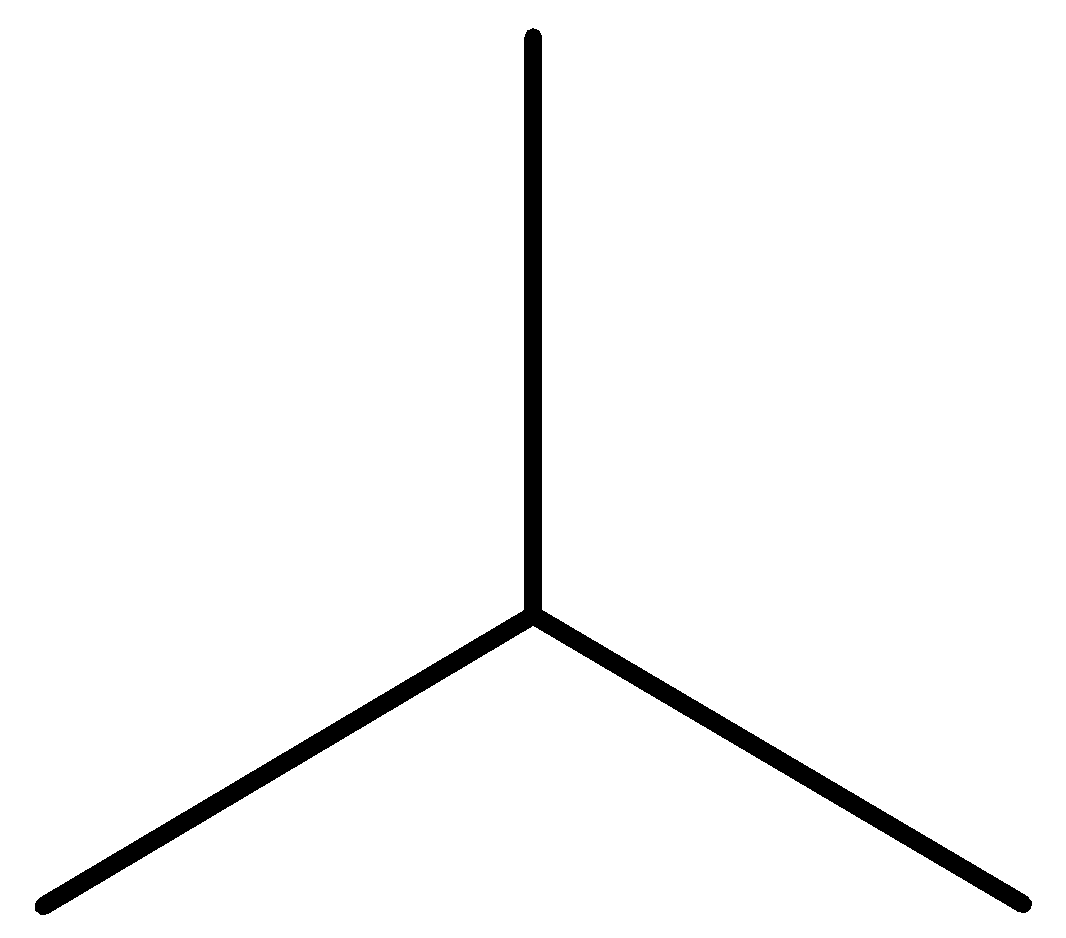
Figure: Structural formula of iso-butane
Note:
The simplest hydrocarbons with all \[C - C\] bonds are alkanes. That is the reason they are called saturated hydrocarbons. The general formula for alkanes is ${C_n}{H_{2n + 2}}$ . In alkanes. all carbon atoms tend to complete their tetra valency by bonding with the same or different atoms and all the carbon atoms form single covalent bonds with other carbon atoms. The parent chain can be branched or unbranched and on the basis of that chemical and physical properties change. Alkanes are comparatively less reactive than hydrocarbons like alkenes, alkynes etc. because all carbon atoms are bonded with single covalent bonds in alkanes which are strong and less reactive in comparison to double or triple covalent bonds of alkenes and alkynes respectively.
${C_4}{H_{10}}$ has only two isomers - n-butane and isobutene.
Complete step by step answer:
Butane is an alkane with four carbon atoms. Its molecular formula is ${C_4}{H_{10}}$ . Its two isomers are n-butane and isobutane or 2 - methylpropane.
n-butane: n butane or normal butane or butane is a colourless gas with a faint petroleum like odour. Its average mass is 58.122 Da and monoisotopic mass is 58.07251 Da.


Figure: Structure formula of n-butane
Iso-butane: The other isomer is iso-butene or 2-methyl propane or i-butane. Iso-butane has a propane parent chain with a methyl group \[-CH3\] attached to the second carbon of the chain. That is why its IUPAC name is 2-methyl propane.


Figure: Structural formula of iso-butane
Note:
The simplest hydrocarbons with all \[C - C\] bonds are alkanes. That is the reason they are called saturated hydrocarbons. The general formula for alkanes is ${C_n}{H_{2n + 2}}$ . In alkanes. all carbon atoms tend to complete their tetra valency by bonding with the same or different atoms and all the carbon atoms form single covalent bonds with other carbon atoms. The parent chain can be branched or unbranched and on the basis of that chemical and physical properties change. Alkanes are comparatively less reactive than hydrocarbons like alkenes, alkynes etc. because all carbon atoms are bonded with single covalent bonds in alkanes which are strong and less reactive in comparison to double or triple covalent bonds of alkenes and alkynes respectively.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




