
Write IUPAC name of the following compound: neopentyl bromide.
Answer
581.7k+ views
Hint: First draw the structure of the compound according to the given common name. Neo is used when three methyl groups are attached to the single carbon atom and iso is used when two methyl groups are attached.
Complete answer:
First let us understand some concepts related to this question:
a) Normal or “n”: It is used when all carbons form a continuous, unbranched (linear) chain.
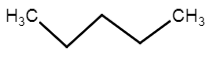
Common Name: n-pentane
IUPAC Name: Pentane
b) Iso prefix is used when all carbons form a continuous chain except one.
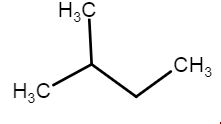
Common Name: iso-pentane
IUPAC name: 2-methyl butane
c) Neo prefix is used when three methyl groups are attached.
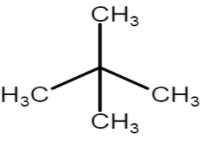
Common Name: iso - pentyl
IUPAC name: 2,2 dimethylpropane
Now we are clear with these prefixes so let’s draw the structure now.
Neopentyl bromide:
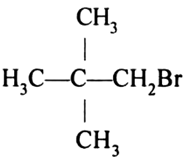
Now keep in mind these important points while IUPAC naming:
- Find and name the longest carbon chain.
- Identify and name the groups which are attached to these chains.
- Now start numbering the chain consecutively, starting at the end nearest to the substituent group.
- Designate the location of each substituent group by an appropriate number and name.
- Now arrange the name, listing group according to alphabetical order.
- Halogen substituent as in this question can be easily named. By using the names like fluoro, chloro, bromo ,iodo.
Now we are clear with all rules, let's name the compound.
We will start numbering from the bromo group. There are two methyl groups attached to 2 carbon.
IUPAC Name:
1 Bromo-2, 2-dimethylpropane.
Note: Don’t forget to arrange the names in alphabetical order. Use words like di ,tri ,tetra for more than one group. Always choose the longest chain as the parent chain.
Complete answer:
First let us understand some concepts related to this question:
a) Normal or “n”: It is used when all carbons form a continuous, unbranched (linear) chain.

Common Name: n-pentane
IUPAC Name: Pentane
b) Iso prefix is used when all carbons form a continuous chain except one.

Common Name: iso-pentane
IUPAC name: 2-methyl butane
c) Neo prefix is used when three methyl groups are attached.

Common Name: iso - pentyl
IUPAC name: 2,2 dimethylpropane
Now we are clear with these prefixes so let’s draw the structure now.
Neopentyl bromide:

Now keep in mind these important points while IUPAC naming:
- Find and name the longest carbon chain.
- Identify and name the groups which are attached to these chains.
- Now start numbering the chain consecutively, starting at the end nearest to the substituent group.
- Designate the location of each substituent group by an appropriate number and name.
- Now arrange the name, listing group according to alphabetical order.
- Halogen substituent as in this question can be easily named. By using the names like fluoro, chloro, bromo ,iodo.
Now we are clear with all rules, let's name the compound.
We will start numbering from the bromo group. There are two methyl groups attached to 2 carbon.
IUPAC Name:
1 Bromo-2, 2-dimethylpropane.
Note: Don’t forget to arrange the names in alphabetical order. Use words like di ,tri ,tetra for more than one group. Always choose the longest chain as the parent chain.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




