
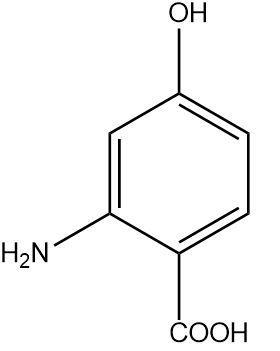
Write IUPAC name of the following compound:

Answer
509.7k+ views
Hint: The IUPAC nomenclature of organic compounds is based on two important rules:
-The priority order of functional groups must be taken in account
-The alphabetic order of naming different functional groups and substituents should be kept in mind.
Complete answer:
The IUPAC nomenclature refers to the systematic method of naming chemical compounds given by the International Union of Pure and Applied Chemistry.
The major rules used in naming organic compounds can be written as follows:
-Find the longest carbon chain that consists of the maximum number of carbons arranged in a straight chain or ring which is called the parent chain.
-Assign priorities to various functional groups and substituents attached to the parent chain.
-The priorities of substitutes are based on the alphabetic order of their names.
-Mention the positions occupied by each functional group or substituent in the compound.
-The naming is done from a side which assigns the lowest positions to all groups attached.
The given organic compound contains a six-membered aromatic ring named benzene. Hence the parent chain in the given organic compound will be benzene and the naming will be done according to it.
There are three different functional groups attached to the benzene ring namely carboxylic acid group $ ( - COOH) $ , amino group $ ( - N{H_2}) $ and an alcohol or hydroxyl group $ ( - OH) $ .
The priority order of various function groups is given as follows:
$ ( - COOH) > ( - S{O_3}H) > ( - COOR) > ( - COCl) > ( - CON{H_2}) > ( - CN) > (HC = O) > ( - CO) > ( - OH) > ( - N{H_2}) $
Hence the carboxylic acid will be assigned the first position. The numbering will be done in a clockwise manner to assign the lowest possible positions. Therefore the second position will be occupied by the amino group and the fourth position will be occupied by the hydroxyl group.
Since $ ( - COOH) $ group has the highest priority, the compound will be named benzoic acid or systematically named benzenecarboxylic acid. On alphabetically naming the remaining functional groups we get 2-amino,4-hydroxy benzenecarboxylic acid.
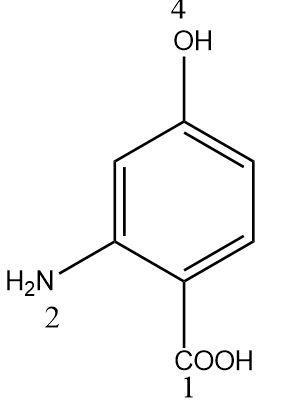
Thus the IUPAC name of the given compound is 2-amino,4-hydroxy benzenecarboxylic acid.
Note:
Once the first priority is assigned to a particular functional group like carboxylic acid in this case, then the numbering of the remaining functional groups is done relative to that of the prior group and not according to the functional group priority order.
-The priority order of functional groups must be taken in account
-The alphabetic order of naming different functional groups and substituents should be kept in mind.
Complete answer:
The IUPAC nomenclature refers to the systematic method of naming chemical compounds given by the International Union of Pure and Applied Chemistry.
The major rules used in naming organic compounds can be written as follows:
-Find the longest carbon chain that consists of the maximum number of carbons arranged in a straight chain or ring which is called the parent chain.
-Assign priorities to various functional groups and substituents attached to the parent chain.
-The priorities of substitutes are based on the alphabetic order of their names.
-Mention the positions occupied by each functional group or substituent in the compound.
-The naming is done from a side which assigns the lowest positions to all groups attached.
The given organic compound contains a six-membered aromatic ring named benzene. Hence the parent chain in the given organic compound will be benzene and the naming will be done according to it.
There are three different functional groups attached to the benzene ring namely carboxylic acid group $ ( - COOH) $ , amino group $ ( - N{H_2}) $ and an alcohol or hydroxyl group $ ( - OH) $ .
The priority order of various function groups is given as follows:
$ ( - COOH) > ( - S{O_3}H) > ( - COOR) > ( - COCl) > ( - CON{H_2}) > ( - CN) > (HC = O) > ( - CO) > ( - OH) > ( - N{H_2}) $
Hence the carboxylic acid will be assigned the first position. The numbering will be done in a clockwise manner to assign the lowest possible positions. Therefore the second position will be occupied by the amino group and the fourth position will be occupied by the hydroxyl group.
Since $ ( - COOH) $ group has the highest priority, the compound will be named benzoic acid or systematically named benzenecarboxylic acid. On alphabetically naming the remaining functional groups we get 2-amino,4-hydroxy benzenecarboxylic acid.

Thus the IUPAC name of the given compound is 2-amino,4-hydroxy benzenecarboxylic acid.
Note:
Once the first priority is assigned to a particular functional group like carboxylic acid in this case, then the numbering of the remaining functional groups is done relative to that of the prior group and not according to the functional group priority order.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




