
Write the balanced chemical equations for the following reactions:
Sodium hydrogen carbonate on reaction with hydrochloric acid gives sodium chloride, water and liberates carbon dioxide.
A. \[NaHC{{O}_{3}}+2HCl\to NaCl+2{{H}_{2}}O+C{{O}_{2}}\]
B. \[2NaHC{{O}_{3}}+2HCl\to NaCl+{{H}_{2}}O+2C{{O}_{2}}\]
C. \[NaHC{{O}_{3}}+HCl\to NaCl+{{H}_{2}}O+C{{O}_{2}}\]
D. \[2NaHC{{O}_{3}}+2HCl\to 2NaCl+{{H}_{2}}O+C{{O}_{2}}\]
Answer
606.3k+ views
Hint: To solve this question, use the law of conservation of mass. Use hit and trial method to balance the number of atoms on both sides.
Complete step by step answer:
Let us solve this question by understanding a chemical equation and the law of conservation of mass.
“A chemical equation shows us the substances involved in a chemical reaction - the substances that react (reactants) and the substances that are produced (products)”.
“The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants”.
According to the question, we’ve been given
Reactants –
Sodium hydrogen carbonate
Hydrochloric acid
Products –
Sodium chloride
Water
Carbon dioxide
Now, we need to balance the number of atoms on the reactants and products side of the reaction.
Let us write the equation first.
\[NaHC{{O}_{3}}+HCl\to NaCl+{{H}_{2}}O+C{{O}_{2}}\]
Let us balance elements on both sides –
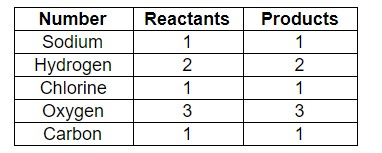
As we can see, the number of elements both reactants and products are balanced.
Therefore, the answer is – option (c) – \[NaHC{{O}_{3}}+HCl\to NaCl+{{H}_{2}}O+C{{O}_{2}}\].
Note: Example of law of conservation of mass –
Combustion process: burning of wood follows conservation of mass since the burning of wood involves Oxygen, Carbon dioxide, water vapor and ashes. The elements in reactants and products are balanced.
Complete step by step answer:
Let us solve this question by understanding a chemical equation and the law of conservation of mass.
“A chemical equation shows us the substances involved in a chemical reaction - the substances that react (reactants) and the substances that are produced (products)”.
“The law of conservation of mass states that mass in an isolated system is neither created nor destroyed by chemical reactions or physical transformations. According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants”.
According to the question, we’ve been given
Reactants –
Sodium hydrogen carbonate
Hydrochloric acid
Products –
Sodium chloride
Water
Carbon dioxide
Now, we need to balance the number of atoms on the reactants and products side of the reaction.
Let us write the equation first.
\[NaHC{{O}_{3}}+HCl\to NaCl+{{H}_{2}}O+C{{O}_{2}}\]
Let us balance elements on both sides –

As we can see, the number of elements both reactants and products are balanced.
Therefore, the answer is – option (c) – \[NaHC{{O}_{3}}+HCl\to NaCl+{{H}_{2}}O+C{{O}_{2}}\].
Note: Example of law of conservation of mass –
Combustion process: burning of wood follows conservation of mass since the burning of wood involves Oxygen, Carbon dioxide, water vapor and ashes. The elements in reactants and products are balanced.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




