
Write the bond line formula and IUPAC name of the following alkynes:
(A) isopropyl acetylene
(B) ethyl isopropyl acetylene
(C) tert butyl methyl acetylene
(D) sec butyl methyl acetylene
Answer
549k+ views
Hint: To answer this question, you should recall the concept of structural and molecular formulas. Use the rules of IUPAC nomenclature to answer this question. The substituent groups are attached to the parent chain and are written as a prefix in the name.
Complete step by step solution:
The electron dot structural formula depiction uses dots to signify the electrons involved with the bonding amid different atoms. The line-bond structural formula is a very frequently used depiction of the structural formula. As the term puts forward, the line-bond structural formula makes use of lines and bonds to show the covalent bonds between atoms. A structural formula displays how the atoms are organized and attached in a molecular formula of a chemical compound. According to the Guidelines set by IUPAC, the nomenclature of compounds must follow these steps:
-The Longest Chain Rule
-The Lowest Set of Locants: Numbering of parent chains in which the lowest number is assigned to the carbon atom which carries the substituents.
Multiple instances of the same substituent
The naming of different substituents
Correct naming of different substituents in case they are present at the same positions
Naming Complex Substituents
Let us apply the above rules on the options systematically
A
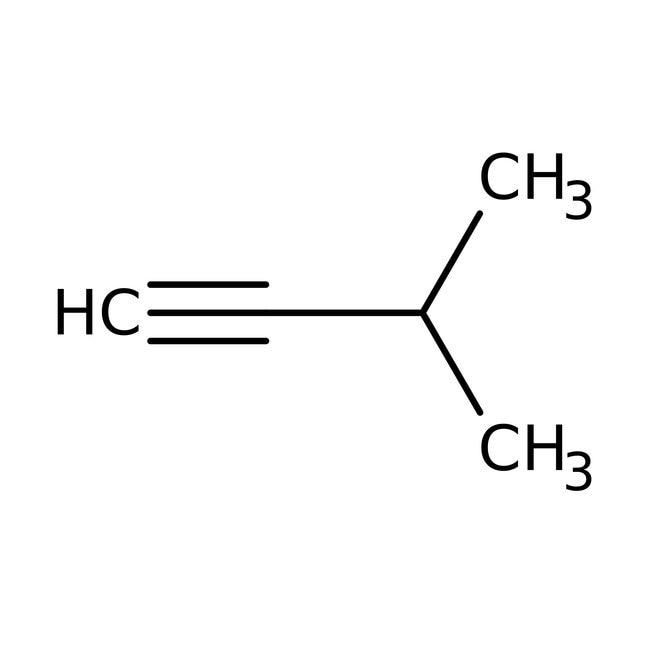
3-Methylbut-1-yne
B
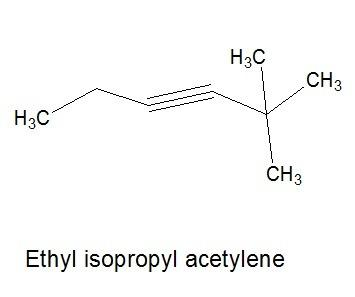
2,2-dimethylhex-3-yne
C
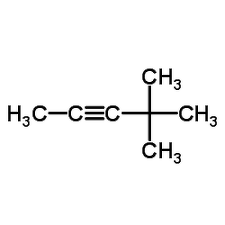
4,4- Dimethyl -2-pentyne
D
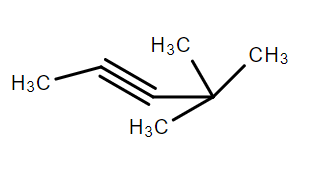
4,4-Dimethyl -pent 2-yne
Note:
While naming of any compound you should be aware of different types of isomerism and their naming. We know that isomers are defined as the molecules with the same molecular formula but possess a different arrangement of the atoms in space or different connectivity of atoms. The phenomenon in the molecules in which the atoms that form the isomers are connected differently is known as structural isomerism. The phenomenon in which the connectivity of atoms is the same in isomers but a different spatial arrangement is a stereoisomerism.
Complete step by step solution:
The electron dot structural formula depiction uses dots to signify the electrons involved with the bonding amid different atoms. The line-bond structural formula is a very frequently used depiction of the structural formula. As the term puts forward, the line-bond structural formula makes use of lines and bonds to show the covalent bonds between atoms. A structural formula displays how the atoms are organized and attached in a molecular formula of a chemical compound. According to the Guidelines set by IUPAC, the nomenclature of compounds must follow these steps:
-The Longest Chain Rule
-The Lowest Set of Locants: Numbering of parent chains in which the lowest number is assigned to the carbon atom which carries the substituents.
Multiple instances of the same substituent
The naming of different substituents
Correct naming of different substituents in case they are present at the same positions
Naming Complex Substituents
Let us apply the above rules on the options systematically
A

3-Methylbut-1-yne
B

2,2-dimethylhex-3-yne
C

4,4- Dimethyl -2-pentyne
D

4,4-Dimethyl -pent 2-yne
Note:
While naming of any compound you should be aware of different types of isomerism and their naming. We know that isomers are defined as the molecules with the same molecular formula but possess a different arrangement of the atoms in space or different connectivity of atoms. The phenomenon in the molecules in which the atoms that form the isomers are connected differently is known as structural isomerism. The phenomenon in which the connectivity of atoms is the same in isomers but a different spatial arrangement is a stereoisomerism.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




