
Write the chemical formula of
1) calcium sulphate
2) Aluminium phosphate
3) Aluminium sulphate
Answer
591.9k+ views
Hint: It a shows the elements which the compound contains and the numbers of atoms of each element in one mole of that compound. There are some rules of writing the chemical formulae of the compounds. Chemical formulae can be said as symbolic representations of chemical compounds.
Complete answer:
Binary compounds made up of two different elements only. Formulae of such compounds can be written using valencies since both types of atom forming the compound must have lost or gained the electrons.
Let us see the rules of writing chemical formulae then we will apply these to write them.
(1) Determine the valencies of the two elements present. Valency is the number of chemical bonds that an element can form.
(2) Write the symbols of elements and chemical formula, so that the sum of the valencies of the two elements are equal. Do this by crossing the charge.
(3) If a metal is present, always place it first in the formula.
Let us start writing by compound 1) Calcium sulphate: The symbol of calcium is $\text{Ca}$ and sulphate is $\text{SO}_{4}^{2-}$. The valency of calcium is 2 as its configuration is $\left( 2,8,8,2 \right)$. The charge on sulphate is -2. Now using the crossing the charge method, the chemical formula will be
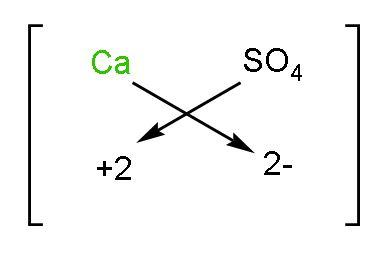
The formula of calcium sulphate will be $\text{C}{{\text{a}}_{2}}{{\left( \text{S}{{\text{O}}_{4}} \right)}_{2}}$ which in simplest form will be $\text{CaS}{{\text{O}}_{4}}$.
2) Aluminium phosphate: The symbol of Aluminium is $\text{Al}$ and phosphate is$\text{PO}_{4}^{3-}$ . The valency of aluminium is 3 as its configuration is $\left( 2,8,3 \right)$. The charge on phosphate is -3. Now using the crossing the charge method, the chemical formula will be
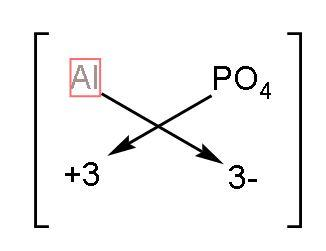
The formula of aluminium phosphate will be $\text{A}{{\text{l}}_{3}}{{\left( \text{P}{{\text{O}}_{4}} \right)}_{3}}$ which in simplest form will be $\text{AlP}{{\text{O}}_{4}}$.
3) Aluminium sulphate: The symbol of Aluminium is $\text{Al}$ and sulphate is $\text{SO}_{4}^{2-}$. The valency of aluminium is 3 as its configuration is $\left( 2,8,3 \right)$. The charge on sulphate is -2. Now using the crossing the charge method, the
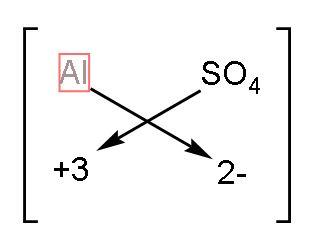
The formula of aluminium sulphate will be $\text{A}{{\text{l}}_{2}}{{\left( \text{S}{{\text{O}}_{4}} \right)}_{3}}$.
Note: After knowing what chemical formulae are, the next question that comes to our minds is; why is there a need for chemical formulae? The answer to this question is
(1) To write chemical equations all the time and it would take too long to write and read, if we use words. So, it is easier to use symbols. Each element has a symbol. The particular way of writing using symbols is called a chemical formula.
(2) In chemical formulae, all the elements that formed the compound and use a small number to the bottom right of an element’s symbol to show the number of atoms of that element are present in the compound. Like the chemical formula for water is ${{\text{H}}_{2}}\text{O}$. It represents a water molecule that has two hydrogen atoms and one oxygen atom.
Complete answer:
Binary compounds made up of two different elements only. Formulae of such compounds can be written using valencies since both types of atom forming the compound must have lost or gained the electrons.
Let us see the rules of writing chemical formulae then we will apply these to write them.
(1) Determine the valencies of the two elements present. Valency is the number of chemical bonds that an element can form.
(2) Write the symbols of elements and chemical formula, so that the sum of the valencies of the two elements are equal. Do this by crossing the charge.
(3) If a metal is present, always place it first in the formula.
Let us start writing by compound 1) Calcium sulphate: The symbol of calcium is $\text{Ca}$ and sulphate is $\text{SO}_{4}^{2-}$. The valency of calcium is 2 as its configuration is $\left( 2,8,8,2 \right)$. The charge on sulphate is -2. Now using the crossing the charge method, the chemical formula will be

The formula of calcium sulphate will be $\text{C}{{\text{a}}_{2}}{{\left( \text{S}{{\text{O}}_{4}} \right)}_{2}}$ which in simplest form will be $\text{CaS}{{\text{O}}_{4}}$.
2) Aluminium phosphate: The symbol of Aluminium is $\text{Al}$ and phosphate is$\text{PO}_{4}^{3-}$ . The valency of aluminium is 3 as its configuration is $\left( 2,8,3 \right)$. The charge on phosphate is -3. Now using the crossing the charge method, the chemical formula will be

The formula of aluminium phosphate will be $\text{A}{{\text{l}}_{3}}{{\left( \text{P}{{\text{O}}_{4}} \right)}_{3}}$ which in simplest form will be $\text{AlP}{{\text{O}}_{4}}$.
3) Aluminium sulphate: The symbol of Aluminium is $\text{Al}$ and sulphate is $\text{SO}_{4}^{2-}$. The valency of aluminium is 3 as its configuration is $\left( 2,8,3 \right)$. The charge on sulphate is -2. Now using the crossing the charge method, the

The formula of aluminium sulphate will be $\text{A}{{\text{l}}_{2}}{{\left( \text{S}{{\text{O}}_{4}} \right)}_{3}}$.
Note: After knowing what chemical formulae are, the next question that comes to our minds is; why is there a need for chemical formulae? The answer to this question is
(1) To write chemical equations all the time and it would take too long to write and read, if we use words. So, it is easier to use symbols. Each element has a symbol. The particular way of writing using symbols is called a chemical formula.
(2) In chemical formulae, all the elements that formed the compound and use a small number to the bottom right of an element’s symbol to show the number of atoms of that element are present in the compound. Like the chemical formula for water is ${{\text{H}}_{2}}\text{O}$. It represents a water molecule that has two hydrogen atoms and one oxygen atom.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




