
Write the complete structural formula.


Answer
573.3k+ views
Hint: We can see that the given structure of the organic compound is made up of four carbons and six hydrogen atoms. Because of the reason it is made up of only hydrogen and carbons , it is known as hydrocarbon. It has two double bonds so it is an alkene.
Complete step by step solution:
As we know that the $IUPAC$nomenclature of the organic compound is $1,3 -\text{butadiene}$. It has four carbons and six hydrogen. The molecular formula of the given formula is $C{H_2}CHCHC{H_2}$. It can be written as ${C_4}{H_6}$. The structural formula of $1,3 - \text{butadiene}$ can be represented as the given figure.
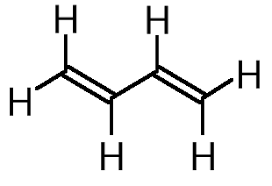
Additional information:
We know that the molecular mass of $C{H_2}CHCHC{H_2}$is $(12 \times 4) + (1 \times 6) = 54g/mol$. $C{H_2}CHCHC{H_2}$ is an important organic compound produced in the process of petroleum. The majority of the $C{H_2}CHCHC{H_2}$ produced in the world is used to manufacture synthetic rubber. It is a colorless gas that can be easily condensed to liquid. According to studies and research it is found and proved that $1,3 -\text{butadiene}$ can cause cancer to human beings. So it is said to be human carcinogen. It can cause cancer in the stomach, blood and lymphatic system. It is soluble in water and alcohol. The density of $1,3 - \text{butadiene}$ is $615Kg/{m^3}$.
Note: Always remember that when an organic compound is made up of only carbons and hydrogens atoms then it is said to be a hydrocarbon. The $IUPAC$ nomenclature of $C{H_2}CHCHC{H_2}$ is $1,3 - \text{butadiene}$. It has a mild gasoline odor. It is used to make tires, resins and plastics. It can cause cancer in human beings. The boiling point of the organic compound $1,3 - \text{butadiene}$ is $ - 4.4^\circ C$.$1,3 -\text{butadiene}$ is made by the union of two vinyl groups.
Complete step by step solution:
As we know that the $IUPAC$nomenclature of the organic compound is $1,3 -\text{butadiene}$. It has four carbons and six hydrogen. The molecular formula of the given formula is $C{H_2}CHCHC{H_2}$. It can be written as ${C_4}{H_6}$. The structural formula of $1,3 - \text{butadiene}$ can be represented as the given figure.

Additional information:
We know that the molecular mass of $C{H_2}CHCHC{H_2}$is $(12 \times 4) + (1 \times 6) = 54g/mol$. $C{H_2}CHCHC{H_2}$ is an important organic compound produced in the process of petroleum. The majority of the $C{H_2}CHCHC{H_2}$ produced in the world is used to manufacture synthetic rubber. It is a colorless gas that can be easily condensed to liquid. According to studies and research it is found and proved that $1,3 -\text{butadiene}$ can cause cancer to human beings. So it is said to be human carcinogen. It can cause cancer in the stomach, blood and lymphatic system. It is soluble in water and alcohol. The density of $1,3 - \text{butadiene}$ is $615Kg/{m^3}$.
Note: Always remember that when an organic compound is made up of only carbons and hydrogens atoms then it is said to be a hydrocarbon. The $IUPAC$ nomenclature of $C{H_2}CHCHC{H_2}$ is $1,3 - \text{butadiene}$. It has a mild gasoline odor. It is used to make tires, resins and plastics. It can cause cancer in human beings. The boiling point of the organic compound $1,3 - \text{butadiene}$ is $ - 4.4^\circ C$.$1,3 -\text{butadiene}$ is made by the union of two vinyl groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




