
Write the electron dot structure of the ethene molecule $\text{ }{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\text{ }$ .
Answer
573.6k+ views
Hint: Lewis dot structure or the electron dot structure is a way of representation of valence electrons in the atom. These valence electrons are represented by the dot around the symbol of the element. The total number of valence electrons is equal to the number of dots in the structure.to determine the electron dot structure, determine the central atom (atoms which have high valence number and high electronegativity) and surrounded by the atom which has a low electronegativity. The ethene has a molecular formula as $\text{ }{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\text{ }$ or $\,\text{C}{{\text{H}}_{\text{2}}}\text{=C}{{\text{H}}_{\text{2}}}\text{ }$ .
Complete answer:
Let's draw a Lewis dot structure for ethane molecules.
Ethene is a saturated hydrocarbon. From its name it is clear that ethene has two carbon atoms. Each carbon is $\,\text{s}{{\text{p}}^{\text{2}}}\text{ }$ hybridized and the molecular structure is$\,\text{C}{{\text{H}}_{\text{2}}}\text{=C}{{\text{H}}_{\text{2}}}\text{ }$.
Follows the below step to determine the Lewis dot structure of the ethene
Step 1) in the first step determines the total number of valence electrons in the carbon atom. the electronic configuration of carbon is as follows,
$\text{ C = 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{2}}}\text{ }$
Here, carbon has a 4 valence electron.
Similarly, the electronic configuration of hydrogen is, $\text{ H = 1}{{\text{s}}^{\text{1}}}\text{ }$
Here, carbon has a 1valence electron and requires one electron to attain the nearest noble gas configuration.
Step 2) in this step, determine the number of electrons required by each atom to attain the nearest Noble gas configuration. The valence shell of carbon contains 4 electrons and thus it requires 4 more electrons to attain the nearest Noble gas configuration of neon. Hydrogen atoms require one more electron to attain the Noble gas configuration of helium.
Step 3) Determine the number of bonds in the molecule.
A covalent bond is formed when each atom shares an electron to form an electron pair. From step 1 and step 2 we know that the number of electrons needed to complete the octet. By subtracting the number of electrons required from the valence electrons we get the number of electrons required for the completion of the octet.
$\text{ No}\text{. of }{{\text{e}}^{-}}\text{ for octet completion = Total electrons in octet }-\text{ Valence electrons of the atoms}$
Thus carbon requires 4 electrons and makes four bonds and hydrogen makes one bond.
Step 4) choose a central atom for the Lewis structure of the molecule.
The central atom is usually an electronegative atom and the atom which has the highest valence number. Here, carbon is more electronegative than the hydrogen atom and it has a high valence number (4) than hydrogen (1). Thus, carbon is the central atom and surrounded by hydrogen atoms.
Step 5) Draw a skeletal structure of a molecule.
Here, we have two carbon atoms that are $\text{ s}{{\text{p}}^{\text{2}}}\text{ }$ hybridized. Thus each carbon requires to make 4 bonds with the surrounding atom. The carbon forms a covalent bond with the other carbon atoms. Here, two carbon atoms share two of its electrons with the neighbouring carbon atom. This leaves each carbon with two electrons. Two-carbon atoms bond to the two hydrogen atoms such that each carbon atom completes its octet. The skeleton for ethene is as shown below,
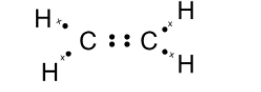
Here, the dot represents the electrons of the carbon atom and x are the electrons of the hydrogen atom. the electrons dot structure or the Lewis dot structure of the ethene is also given as,
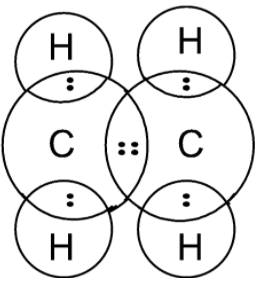
Note: The Lewis dot structure does not explain the geometry of molecules or how the Bonds are formed, or how the electrons are shared between the atoms. The Lewis dot structure is a simple and limited theory based on the electronic structure and valence numbers.
Complete answer:
Let's draw a Lewis dot structure for ethane molecules.
Ethene is a saturated hydrocarbon. From its name it is clear that ethene has two carbon atoms. Each carbon is $\,\text{s}{{\text{p}}^{\text{2}}}\text{ }$ hybridized and the molecular structure is$\,\text{C}{{\text{H}}_{\text{2}}}\text{=C}{{\text{H}}_{\text{2}}}\text{ }$.
Follows the below step to determine the Lewis dot structure of the ethene
Step 1) in the first step determines the total number of valence electrons in the carbon atom. the electronic configuration of carbon is as follows,
$\text{ C = 1}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{s}}^{\text{2}}}\text{ 2}{{\text{p}}^{\text{2}}}\text{ }$
Here, carbon has a 4 valence electron.
Similarly, the electronic configuration of hydrogen is, $\text{ H = 1}{{\text{s}}^{\text{1}}}\text{ }$
Here, carbon has a 1valence electron and requires one electron to attain the nearest noble gas configuration.
Step 2) in this step, determine the number of electrons required by each atom to attain the nearest Noble gas configuration. The valence shell of carbon contains 4 electrons and thus it requires 4 more electrons to attain the nearest Noble gas configuration of neon. Hydrogen atoms require one more electron to attain the Noble gas configuration of helium.
Step 3) Determine the number of bonds in the molecule.
A covalent bond is formed when each atom shares an electron to form an electron pair. From step 1 and step 2 we know that the number of electrons needed to complete the octet. By subtracting the number of electrons required from the valence electrons we get the number of electrons required for the completion of the octet.
$\text{ No}\text{. of }{{\text{e}}^{-}}\text{ for octet completion = Total electrons in octet }-\text{ Valence electrons of the atoms}$
Thus carbon requires 4 electrons and makes four bonds and hydrogen makes one bond.
Step 4) choose a central atom for the Lewis structure of the molecule.
The central atom is usually an electronegative atom and the atom which has the highest valence number. Here, carbon is more electronegative than the hydrogen atom and it has a high valence number (4) than hydrogen (1). Thus, carbon is the central atom and surrounded by hydrogen atoms.
Step 5) Draw a skeletal structure of a molecule.
Here, we have two carbon atoms that are $\text{ s}{{\text{p}}^{\text{2}}}\text{ }$ hybridized. Thus each carbon requires to make 4 bonds with the surrounding atom. The carbon forms a covalent bond with the other carbon atoms. Here, two carbon atoms share two of its electrons with the neighbouring carbon atom. This leaves each carbon with two electrons. Two-carbon atoms bond to the two hydrogen atoms such that each carbon atom completes its octet. The skeleton for ethene is as shown below,
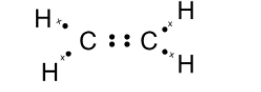
Here, the dot represents the electrons of the carbon atom and x are the electrons of the hydrogen atom. the electrons dot structure or the Lewis dot structure of the ethene is also given as,

Note: The Lewis dot structure does not explain the geometry of molecules or how the Bonds are formed, or how the electrons are shared between the atoms. The Lewis dot structure is a simple and limited theory based on the electronic structure and valence numbers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




