
Write the electronic configuration of the Boron.
Answer
588k+ views
Hint: Boron belongs to the group no. 13 and it’s atomic no. is 5. So, it has 5 electrons. General valence electronic configuration of group 13 elements is $n{s^2}n{p^1}$. To write the electronic configuration of boron, fill its 5 electrons in the orbitals arranged in order of increasing energies, according to Aufbau Principle.
Complete step by step solution:
An electron in an atom is characterised by 4 quantum numbers, and the principal quantum number $(n)$ defines the main energy level known as the shell. Each shell consists of one or more subshells or sub-levels. Each subshell is assigned by azimuthal quantum no. ($l$) and $l$have values from 0 to $n - 1$ Also, each subshell has orbitals equal to \[2l + 1\] . The distribution of electrons into orbitals of an atom is called its electronic configuration.
Boron has atomic no. 5 and it is represented by symbol the B. It belongs to group 13 of p-block. Group 13 elements outermost or valence electronic configuration is \[\left[ E \right]n{s^2}n{p^1}\], where E is an inert gas configuration).
To find the electronic configuration of boron, fill 5 electrons of boron in orbitals according to Aufbau principle. Consequently, electronic configuration of boron will be :\[1{s^2}2{s^2}2{p^1}\].
Now, to write the noble gas electronic configuration of boron, we need to find the noble gas that comes before boron in the periodic table and it is helium ( electronic configuration of helium is \[1{s^2}\]).
Therefore, the noble gas electronic configuration of boron is \[\left[ {He} \right]2{s^2}2{p^1}\]. The 2 in $2{s^2}and\,2{p^2}$ denotes the shell no (n) and shell number denotes the period no. to which the element belongs. Thus, boron belongs to period 2 of the periodic table.
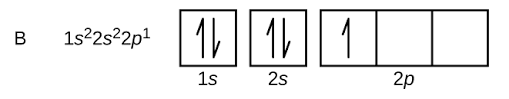
Note: The orbital picture of boron can be represented as :
$s$- orbital can hold up maximum 2 electrons, $p$-orbitals can hold maximum 6 electrons.
Complete step by step solution:
An electron in an atom is characterised by 4 quantum numbers, and the principal quantum number $(n)$ defines the main energy level known as the shell. Each shell consists of one or more subshells or sub-levels. Each subshell is assigned by azimuthal quantum no. ($l$) and $l$have values from 0 to $n - 1$ Also, each subshell has orbitals equal to \[2l + 1\] . The distribution of electrons into orbitals of an atom is called its electronic configuration.
Boron has atomic no. 5 and it is represented by symbol the B. It belongs to group 13 of p-block. Group 13 elements outermost or valence electronic configuration is \[\left[ E \right]n{s^2}n{p^1}\], where E is an inert gas configuration).
To find the electronic configuration of boron, fill 5 electrons of boron in orbitals according to Aufbau principle. Consequently, electronic configuration of boron will be :\[1{s^2}2{s^2}2{p^1}\].
Now, to write the noble gas electronic configuration of boron, we need to find the noble gas that comes before boron in the periodic table and it is helium ( electronic configuration of helium is \[1{s^2}\]).
Therefore, the noble gas electronic configuration of boron is \[\left[ {He} \right]2{s^2}2{p^1}\]. The 2 in $2{s^2}and\,2{p^2}$ denotes the shell no (n) and shell number denotes the period no. to which the element belongs. Thus, boron belongs to period 2 of the periodic table.
Note: The orbital picture of boron can be represented as :
$s$- orbital can hold up maximum 2 electrons, $p$-orbitals can hold maximum 6 electrons.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




