
Write the electrophilic substitution reaction mechanism for benzene with an example.
Answer
564.9k+ views
Hint:Electrophilic substitution reactions are those reactions in which the functional group that is present on the compound is replaced with an electrophile. The functional group that gets removed is hydrogen in most of the cases but it is not compulsory.
Complete answer:
Electrophilic substitution reactions occur by a three-step mechanism that includes the following steps: (a) Generation of an electrophile (b) Formation of a carbocation (an intermediate) and (c) Removal of a proton from the intermediate.
In electrophilic aromatic substitution reactions, the atom which is attached to the ring gets replaced by an electrophile. These reactions include aromatic nitration, sulphonation, and Friedel-Crafts reactions. The aromaticity of benzene does not get disturbed in reaction. Hence the spontaneity of these reactions is very high.
MECHANISM OF ELECTROPHILIC SUBSTITUTION ON BENZENE(NITRATION)
When Benzene is treated with concentrated nitric acid in the presence of concentrated sulphuric acid at \[323 - 333\,K\] to yield nitrobenzene. This electrophilic substitution reaction is known as the nitration of benzene.
STEP-1: Generation of Electrophile
The first step is to activate \[HN{O_3}\] with ${H_2}S{O_4}$ to generate a strong electrophile, the nitronium ion. \[HN{O_3}\] accepts a proton from ${H_2}S{O_4}$ and then it gets dissociated to form the nitronium ion $NO_2^ + $.
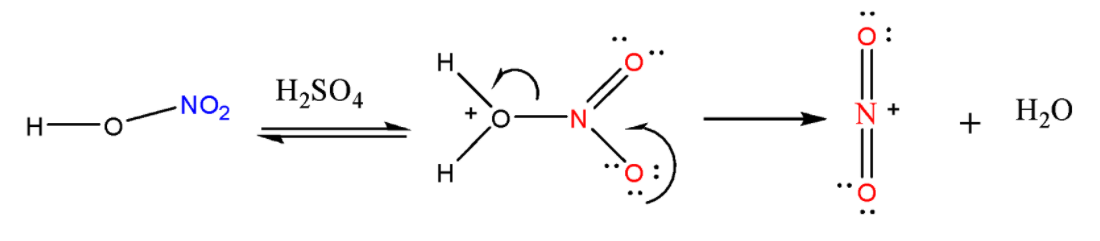
STEP-2: Formation of Arenium ion
The electrophile $NO_2^ + $ that is generated attacks on the benzene ring to form a positively charged cyclohexadienyl cation also known as arenium ion that has one\[\;s{p^3}\;\] hybridized carbon. The positive charge gets distributed over all the three carbon atoms via resonance making the ring partially stable.

STEP-3: Removal of the proton
In the final step arenium ion loses its proton from the \[\;s{p^3}\;\]carbon to Lewis base that results in the formation of nitrobenzene.
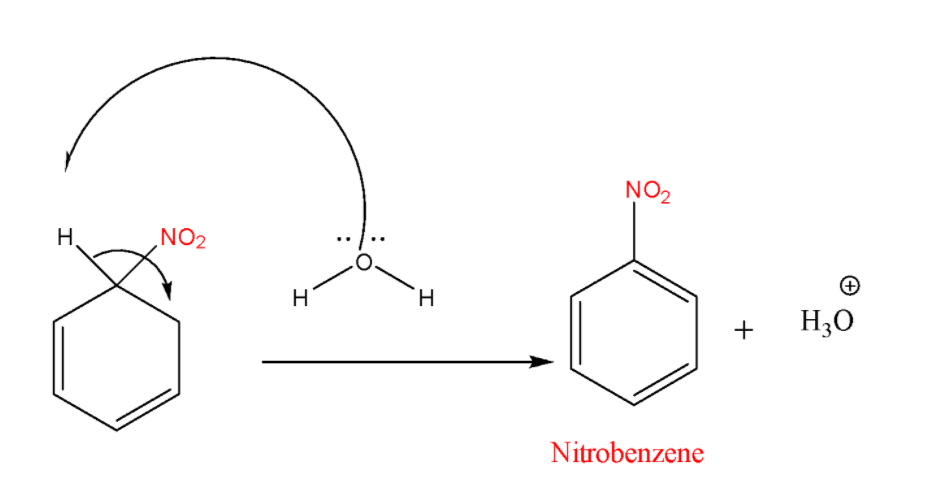
Note:
Electrophiles are defined as electron-deficient species that get attracted to an electron-rich center. They react by accepting an electron pair to get bonded to a nucleophile including the interactions of a proton and a base.
Complete answer:
Electrophilic substitution reactions occur by a three-step mechanism that includes the following steps: (a) Generation of an electrophile (b) Formation of a carbocation (an intermediate) and (c) Removal of a proton from the intermediate.
In electrophilic aromatic substitution reactions, the atom which is attached to the ring gets replaced by an electrophile. These reactions include aromatic nitration, sulphonation, and Friedel-Crafts reactions. The aromaticity of benzene does not get disturbed in reaction. Hence the spontaneity of these reactions is very high.
MECHANISM OF ELECTROPHILIC SUBSTITUTION ON BENZENE(NITRATION)
When Benzene is treated with concentrated nitric acid in the presence of concentrated sulphuric acid at \[323 - 333\,K\] to yield nitrobenzene. This electrophilic substitution reaction is known as the nitration of benzene.
STEP-1: Generation of Electrophile
The first step is to activate \[HN{O_3}\] with ${H_2}S{O_4}$ to generate a strong electrophile, the nitronium ion. \[HN{O_3}\] accepts a proton from ${H_2}S{O_4}$ and then it gets dissociated to form the nitronium ion $NO_2^ + $.

STEP-2: Formation of Arenium ion
The electrophile $NO_2^ + $ that is generated attacks on the benzene ring to form a positively charged cyclohexadienyl cation also known as arenium ion that has one\[\;s{p^3}\;\] hybridized carbon. The positive charge gets distributed over all the three carbon atoms via resonance making the ring partially stable.

STEP-3: Removal of the proton
In the final step arenium ion loses its proton from the \[\;s{p^3}\;\]carbon to Lewis base that results in the formation of nitrobenzene.

Note:
Electrophiles are defined as electron-deficient species that get attracted to an electron-rich center. They react by accepting an electron pair to get bonded to a nucleophile including the interactions of a proton and a base.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




