
Write the empirical formula for acetic acid.
Answer
606.3k+ views
Hint: Having a basic idea about the structure of Acetic Acid will give us an idea how to form the empirical formula. It is even advised to recall the different rules of nomenclature.
Complete step-by-step answer:
Here is the structure of Acetic Acid:
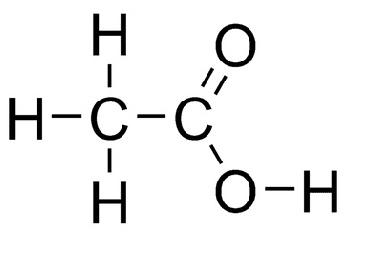
From the structure we can write the formula of Acetic Acid, which is:
${\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}$
In chemistry the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound.
There are 4 hydrogen atoms, 2 carbon atoms and 2 oxygen atoms.
So, we can write the empirical formula of Acetic Acid as:
${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}$
Note: So, here are some properties of acetic acid that we should know:
Acetic acid is important for microorganisms in food industry because of their ability to oxidize many types of sugars and alcohol to organic acids as end product during fermentation process
Acetic acid can be a hazardous chemical if not used in a safe and appropriate manner. This liquid is highly corrosive to the skin and eyes and because of this must be handled with extreme care acetic acid can also be damaging to the internal organs are interested or in the case of vapour inhalation.
Acetic acid is used in vinegar which is used as a condiment and in the pickling of raw vegetables and other foods. Acetic acid is used for the manufacture of inks and dyes.
We should also know that acetic acid is a weak monoprotic acid.
Complete step-by-step answer:
Here is the structure of Acetic Acid:

From the structure we can write the formula of Acetic Acid, which is:
${\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}$
In chemistry the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound.
There are 4 hydrogen atoms, 2 carbon atoms and 2 oxygen atoms.
So, we can write the empirical formula of Acetic Acid as:
${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}$
Note: So, here are some properties of acetic acid that we should know:
Acetic acid is important for microorganisms in food industry because of their ability to oxidize many types of sugars and alcohol to organic acids as end product during fermentation process
Acetic acid can be a hazardous chemical if not used in a safe and appropriate manner. This liquid is highly corrosive to the skin and eyes and because of this must be handled with extreme care acetic acid can also be damaging to the internal organs are interested or in the case of vapour inhalation.
Acetic acid is used in vinegar which is used as a condiment and in the pickling of raw vegetables and other foods. Acetic acid is used for the manufacture of inks and dyes.
We should also know that acetic acid is a weak monoprotic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




