
Write the formula of allyl alcohol and benzyl alcohol.
Answer
585.3k+ views
Hint:
The IUPAC name for allyl alcohol is 2-propen-1-ol.
The IUPAC name for benzyl alcohol is phenylmethanol.
Complete step by step answer:
-Step 1:
-Write the condensed structural formula of allyl alcohol as follows:
-The IUPAC name for allyl alcohol is 2-propen-1-ol. 2-propen suggests that there are three carbon atoms and double bonds at the second carbon. 1-ol suggests that the $ - {\text{OH}}$ functional group is attached to the first carbon. Thus, the condensed structural formula of allyl alcohol is,
${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$
-Write the structural formula of allyl alcohol as follows:
-The structural formula of allyl alcohol is ${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$.

-Write the molecular formula of allyl alcohol as follows:
The condensed structural formula of allyl alcohol is ${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$. It contains three carbon atoms, six hydrogen atoms and one oxygen atom. Thus, the molecular formula of allyl alcohol is,
${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$.
-Step 2:
-Write the condensed structural formula of benzyl alcohol as follows:
-The IUPAC name for benzyl alcohol is phenylmethanol. Phenylmethanol suggests that methanol${\text{C}}{{\text{H}}_2} - {\text{OH}}$ functional group is attached to the phenyl ring. Thus, the condensed structural formula of benzyl alcohol is,
${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$
-Write the structural formula of benzyl alcohol as follows:
-The structural formula of benzyl alcohol is ${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$.
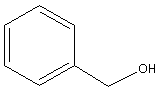
-Write the molecular formula of benzyl alcohol as follows:
-The condensed structural formula of benzyl alcohol is ${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$. It contains seven carbon atoms, eight hydrogen atoms and one oxygen atom. Thus, the molecular formula of benzyl alcohol is,
${{\text{C}}_{\text{7}}}{{\text{H}}_{\text{8}}}{\text{O}}$.
Note: A molecule is represented using a molecular formula. The molecular formula represents the number of atoms of different elements present in molecules.
The structural formula represents which atoms are bonded to each other and the condensed structural formula is condensed form of structural formula.
The IUPAC name for allyl alcohol is 2-propen-1-ol.
The IUPAC name for benzyl alcohol is phenylmethanol.
Complete step by step answer:
-Step 1:
-Write the condensed structural formula of allyl alcohol as follows:
-The IUPAC name for allyl alcohol is 2-propen-1-ol. 2-propen suggests that there are three carbon atoms and double bonds at the second carbon. 1-ol suggests that the $ - {\text{OH}}$ functional group is attached to the first carbon. Thus, the condensed structural formula of allyl alcohol is,
${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$
-Write the structural formula of allyl alcohol as follows:
-The structural formula of allyl alcohol is ${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$.

-Write the molecular formula of allyl alcohol as follows:
The condensed structural formula of allyl alcohol is ${\text{C}}{{\text{H}}_{\text{2}}} = {\text{CH}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$. It contains three carbon atoms, six hydrogen atoms and one oxygen atom. Thus, the molecular formula of allyl alcohol is,
${{\text{C}}_{\text{3}}}{{\text{H}}_{\text{6}}}{\text{O}}$.
-Step 2:
-Write the condensed structural formula of benzyl alcohol as follows:
-The IUPAC name for benzyl alcohol is phenylmethanol. Phenylmethanol suggests that methanol${\text{C}}{{\text{H}}_2} - {\text{OH}}$ functional group is attached to the phenyl ring. Thus, the condensed structural formula of benzyl alcohol is,
${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$
-Write the structural formula of benzyl alcohol as follows:
-The structural formula of benzyl alcohol is ${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$.

-Write the molecular formula of benzyl alcohol as follows:
-The condensed structural formula of benzyl alcohol is ${{\text{C}}_6}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_{\text{2}}} - {\text{OH}}$. It contains seven carbon atoms, eight hydrogen atoms and one oxygen atom. Thus, the molecular formula of benzyl alcohol is,
${{\text{C}}_{\text{7}}}{{\text{H}}_{\text{8}}}{\text{O}}$.
Note: A molecule is represented using a molecular formula. The molecular formula represents the number of atoms of different elements present in molecules.
The structural formula represents which atoms are bonded to each other and the condensed structural formula is condensed form of structural formula.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




