
Write the IUPAC name of the following compound.
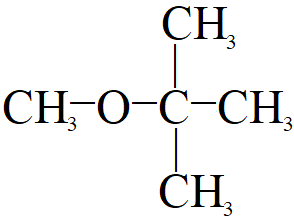
Answer
599.1k+ views
Hint- In order to determine the IUPAC name of given carbon structure first we will understand what is the process to give an IUPAC name of any carbon structure then according to it we will write the IUPAC name of given structure.
Complete answer:
> Here is a short set of guidelines for writing the name of every carbon structure in IUPAC
1) The longest carbon chain is known. This chain is known as the parent line.
2) Identify all substituent classes (attached from the parent chain).
3) Number the parent chain carbons from the end and give the lowest number to the substituent. The series which is the "lowest" when evaluating a set of numbers is the one where the first difference contains the lowest number. If two or more lateral chains are equal, assign the lowest number to the first one in the description.
4) If the same substituent appears more than once, the position for every point where the substituent appears is indicated. Alternatively, the number of times the substituent category occurs is defined by a suffix (di, tri, tetra etc.)
5) If two or more different substituents exist, they are identified in alphabetical order using the base name (the prefixes are neglected).
> Apart from these rules there are some more rules as well.
- Here from the structure we find the number of carbon in the longest chain is 3 so the name will contain “prop” and also the chain with 3 carbon has no double bond or triple bond so, its name on the basis of longest chain will be “propane”.
- The compound has a “methoxy '' group attached to the second carbon from the right or the left. So the prefix will be “2-methoxy”. Also in the similar way the second carbon also has an attached methyl group to its structure so we have to use the prefix “2-methyl“ as the methoxy group has higher priority than methyl group so it will come first.
Hence, the given compound is “2-methoxy-2-methyl-propane”.
Note- The IUPAC nomenclature in organic chemistry is a system in organic chemical compounds in molecular nomenclature, as defined by the International Union of Pure and Applied Chemistry (IUPAC). This is written in the Organic Chemistry Nomenclature (informally called the Blue Book). Ideally a conceivable organic compound should have a name from which to construct an unmistakable structural formula. Some of the rules are listed in the solution, to name a compound, students must remember certain rules.
Complete answer:
> Here is a short set of guidelines for writing the name of every carbon structure in IUPAC
1) The longest carbon chain is known. This chain is known as the parent line.
2) Identify all substituent classes (attached from the parent chain).
3) Number the parent chain carbons from the end and give the lowest number to the substituent. The series which is the "lowest" when evaluating a set of numbers is the one where the first difference contains the lowest number. If two or more lateral chains are equal, assign the lowest number to the first one in the description.
4) If the same substituent appears more than once, the position for every point where the substituent appears is indicated. Alternatively, the number of times the substituent category occurs is defined by a suffix (di, tri, tetra etc.)
5) If two or more different substituents exist, they are identified in alphabetical order using the base name (the prefixes are neglected).
> Apart from these rules there are some more rules as well.
- Here from the structure we find the number of carbon in the longest chain is 3 so the name will contain “prop” and also the chain with 3 carbon has no double bond or triple bond so, its name on the basis of longest chain will be “propane”.
- The compound has a “methoxy '' group attached to the second carbon from the right or the left. So the prefix will be “2-methoxy”. Also in the similar way the second carbon also has an attached methyl group to its structure so we have to use the prefix “2-methyl“ as the methoxy group has higher priority than methyl group so it will come first.
Hence, the given compound is “2-methoxy-2-methyl-propane”.
Note- The IUPAC nomenclature in organic chemistry is a system in organic chemical compounds in molecular nomenclature, as defined by the International Union of Pure and Applied Chemistry (IUPAC). This is written in the Organic Chemistry Nomenclature (informally called the Blue Book). Ideally a conceivable organic compound should have a name from which to construct an unmistakable structural formula. Some of the rules are listed in the solution, to name a compound, students must remember certain rules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




