
How can I write the Lewis dot structure for $ MgC{{l}_{2}} $ ?
Answer
560.7k+ views
Hint The answer is based on the concept of inorganic chemistry which deals with Lewis dot structures that is nothing but the diagrams of a molecule that represent the valence electrons of atoms within the molecule.
Complete step – by – step answer:
In the previous classes of chemistry, we have come across the various notations used to write the structure of the molecules and also have studied how to represent the lone pair of electrons present on the atom and also relating concepts.
We shall see the method to write the Lewis dot structure of the given compound that is magnesium chloride.
- Lewis dot structures which are also called as the electron dot structures are the diagrams that represent the total valence electrons present on that atom in a molecule.
- The valence electrons of the atom can be found by writing their electronic configuration or can also be directly determined based on the atomic number. The outermost electrons present in the atom can be known by this which is nothing but are called the valence electrons.
- Magnesium chloride has the molecular formula where the valence electrons present in magnesium is 2 and that in the chlorine atom is 7. Two electrons each of magnesium are donated to two chlorine atoms each and therefore magnesium gets positive charge of +2.
- The chlorine gains one electron each and has now, total of 8 valence electrons with a negative charge on each of them. Thus, the Lewis structure which is ionic form is as shown below,
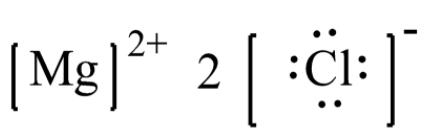
Note: It is important to note the Lewis dot structures helps us to visualize the valence electrons present in the atom of a molecule and also gives information about whether they exist as lone pairs of electrons or whether the electrons are present within the bond. These structures are simply called Lewis structures.
Complete step – by – step answer:
In the previous classes of chemistry, we have come across the various notations used to write the structure of the molecules and also have studied how to represent the lone pair of electrons present on the atom and also relating concepts.
We shall see the method to write the Lewis dot structure of the given compound that is magnesium chloride.
- Lewis dot structures which are also called as the electron dot structures are the diagrams that represent the total valence electrons present on that atom in a molecule.
- The valence electrons of the atom can be found by writing their electronic configuration or can also be directly determined based on the atomic number. The outermost electrons present in the atom can be known by this which is nothing but are called the valence electrons.
- Magnesium chloride has the molecular formula where the valence electrons present in magnesium is 2 and that in the chlorine atom is 7. Two electrons each of magnesium are donated to two chlorine atoms each and therefore magnesium gets positive charge of +2.
- The chlorine gains one electron each and has now, total of 8 valence electrons with a negative charge on each of them. Thus, the Lewis structure which is ionic form is as shown below,
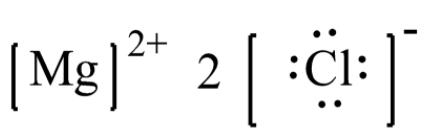
Note: It is important to note the Lewis dot structures helps us to visualize the valence electrons present in the atom of a molecule and also gives information about whether they exist as lone pairs of electrons or whether the electrons are present within the bond. These structures are simply called Lewis structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




