
Write the lewis dot structure of water (${H_2}O$).
Answer
579.6k+ views
Hint: Lewis structures are electron dot formulas. We assemble the molecule or ion from the constituents atoms showing only the valence electrons(i.e., the electrons of the outermost shell).
Step by step answer: We try to give every atom the noble gas electronic configuration in the same horizontal row of the periodic table. This is done to satisfy the octet rule. The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom. Now let us define the rules to write lewis electron dot structure for water:
Firstly, we will find the total number of valence electrons of all the atoms in the water molecule. So the water molecule has 2 H atoms and one O atom which has the valency of one and six electrons respectively. So the total number of valence electrons are 1+6+1=8.
Now we will pair each atom such as to achieve the noble electronic configuration, therefore both the hydrogen atoms share their one electron with oxygen already having six valence electrons giving oxygen noble gas configuration of eight electrons and hence completing its octet. Oxygen also shares one electron each with the hydrogen atom such that it has two electrons and it too achieves noble electronic configuration of helium.
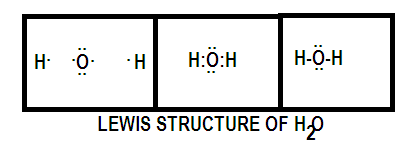
Hence hydrogen forms its duet and oxygen an octet. Now we will form bonds between atoms and represent these bonding pairs with lines.
Note: We need to take care of the fact that if the group structure is an ion, we will add or subtract electrons to give it the proper charge. If necessary we use multiple bonds to satisfy the octet rule to give noble gas configuration.
Step by step answer: We try to give every atom the noble gas electronic configuration in the same horizontal row of the periodic table. This is done to satisfy the octet rule. The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom. Now let us define the rules to write lewis electron dot structure for water:
Firstly, we will find the total number of valence electrons of all the atoms in the water molecule. So the water molecule has 2 H atoms and one O atom which has the valency of one and six electrons respectively. So the total number of valence electrons are 1+6+1=8.
Now we will pair each atom such as to achieve the noble electronic configuration, therefore both the hydrogen atoms share their one electron with oxygen already having six valence electrons giving oxygen noble gas configuration of eight electrons and hence completing its octet. Oxygen also shares one electron each with the hydrogen atom such that it has two electrons and it too achieves noble electronic configuration of helium.

Hence hydrogen forms its duet and oxygen an octet. Now we will form bonds between atoms and represent these bonding pairs with lines.
Note: We need to take care of the fact that if the group structure is an ion, we will add or subtract electrons to give it the proper charge. If necessary we use multiple bonds to satisfy the octet rule to give noble gas configuration.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




