
Write the Lewis dot symbols for atoms of the following elements:
Mg, Na, B, O, N and Br.
Answer
567.6k+ views
Hint: The answer to this question is based on the concept of the Lewis dot structure which represents the total valence electrons of atoms within a molecule. This fact gives you the correct answer.
Complete Solution :
- We have studied in the lower classes in the chapters of inorganic chemistry that deals with various forms of writing the structure of compounds and one among which is the Lewis dot structure and is based on the total valency of electrons in its outermost orbital.
We shall see what the meaning of Lewis dot structure is and then deduce the required answer.
- Lewis dot structure which is also called as electron dot structure is the way of denoting the total number of valence electrons present in the atom or molecule which can undergo further bonding or can remain as lone pair of electrons.
- Now in the given above atoms, we can calculate total valence electrons and write in the form of Lewis dot structure.
- Now, in magnesium, the atomic number is 12 and the electronic configuration of it is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}$ and there are two electrons in the outermost shell and thus it has two valence electrons.
- Similarly in sodium with atomic number 11, the configuration is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}$ and hence have one electron in outermost orbit.
- In case of boron with atomic number 5 have configuration $1{{s}^{2}}2{{s}^{2}}2{{p}^{1}}$ and thus have three electrons in outermost orbit.
The atom oxygen with atomic number 8 has the configuration $1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}$and there are six electrons in the outer orbit among which two pairs are lone pair of electrons
- In case of nitrogen with atomic number 7, the configuration will be $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$ and 5 have outer electrons among which one pair is lone pair.
- In case of bromine with atomic number 35 have configuration $[Ar]4{{s}^{2}}3{{d}^{10}}4{{p}^{5}}$ where the outermost orbit is having 7 electrons.
Therefore, Lewis dot structures for all these atoms can be written as shown below:
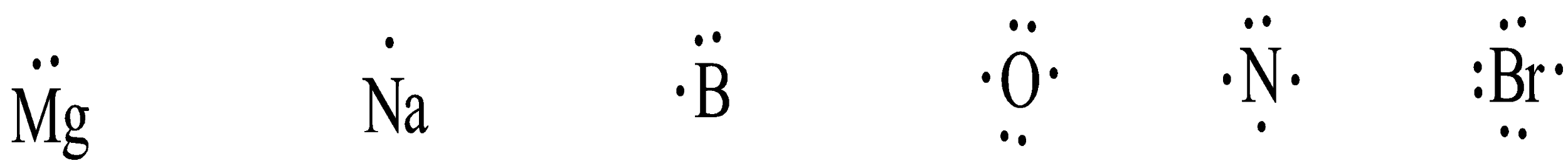
Note: Note that the outermost orbit of an atom is the one which has greater number in numerical and total electrons are to be counted from that orbit and not that of the lower orbit. In $[Ar]4{{s}^{2}}3{{d}^{10}}4{{p}^{5}}$, the highest numerical is 4 and the electrons in that orbit will be 2 + 5 which will be 7 and not all the extreme ends of configuration that is 2 + 10 + 5 = 17.
Complete Solution :
- We have studied in the lower classes in the chapters of inorganic chemistry that deals with various forms of writing the structure of compounds and one among which is the Lewis dot structure and is based on the total valency of electrons in its outermost orbital.
We shall see what the meaning of Lewis dot structure is and then deduce the required answer.
- Lewis dot structure which is also called as electron dot structure is the way of denoting the total number of valence electrons present in the atom or molecule which can undergo further bonding or can remain as lone pair of electrons.
- Now in the given above atoms, we can calculate total valence electrons and write in the form of Lewis dot structure.
- Now, in magnesium, the atomic number is 12 and the electronic configuration of it is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}$ and there are two electrons in the outermost shell and thus it has two valence electrons.
- Similarly in sodium with atomic number 11, the configuration is $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}$ and hence have one electron in outermost orbit.
- In case of boron with atomic number 5 have configuration $1{{s}^{2}}2{{s}^{2}}2{{p}^{1}}$ and thus have three electrons in outermost orbit.
The atom oxygen with atomic number 8 has the configuration $1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}$and there are six electrons in the outer orbit among which two pairs are lone pair of electrons
- In case of nitrogen with atomic number 7, the configuration will be $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$ and 5 have outer electrons among which one pair is lone pair.
- In case of bromine with atomic number 35 have configuration $[Ar]4{{s}^{2}}3{{d}^{10}}4{{p}^{5}}$ where the outermost orbit is having 7 electrons.
Therefore, Lewis dot structures for all these atoms can be written as shown below:
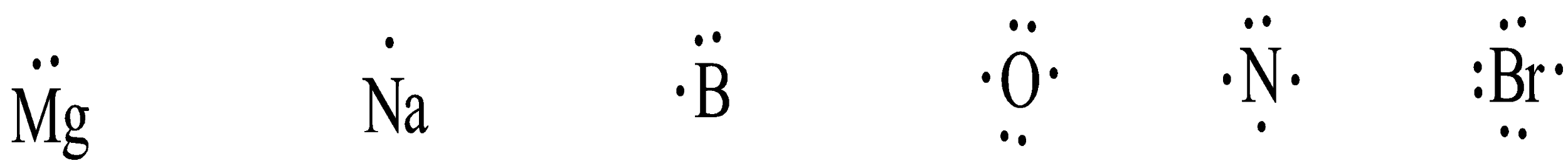
Note: Note that the outermost orbit of an atom is the one which has greater number in numerical and total electrons are to be counted from that orbit and not that of the lower orbit. In $[Ar]4{{s}^{2}}3{{d}^{10}}4{{p}^{5}}$, the highest numerical is 4 and the electrons in that orbit will be 2 + 5 which will be 7 and not all the extreme ends of configuration that is 2 + 10 + 5 = 17.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




