
Write the lewis dot symbols for:
i.${H_2}O$
ii.$CC{l_4}$
iii.${H_2}$
Answer
566.7k+ views
Hint: The simplest notation to represent the valence electron in an atom is known as the lewis dot symbol. Lewis dot structure provides a picture of bonding in molecules and ions in terms of the shared pairs of the electrons and the octet rule.
Complete step by step answer:
The steps needed to be followed to write the lewis dot structure:
Calculate the number of valence electrons of an atom present.
If the given species is an anion then add the number of electrons equal to a unit of negative charge and if the given species is cation then subtract the number of electrons equal to a unit of a positive charge.
Select the central atom and draw the skeleton structure with intelligence guesses.
Remember that hydrogen and fluorine usually occupy the terminal position.
Put the shared pair of electrons between two atoms to represent the single bond and put the remaining electrons to represent multiple bonds or show them as a lone pair.
The lewis dot structure of the given compound is written as:
i.Lewis dot structure of water (${H_2}O$) is:
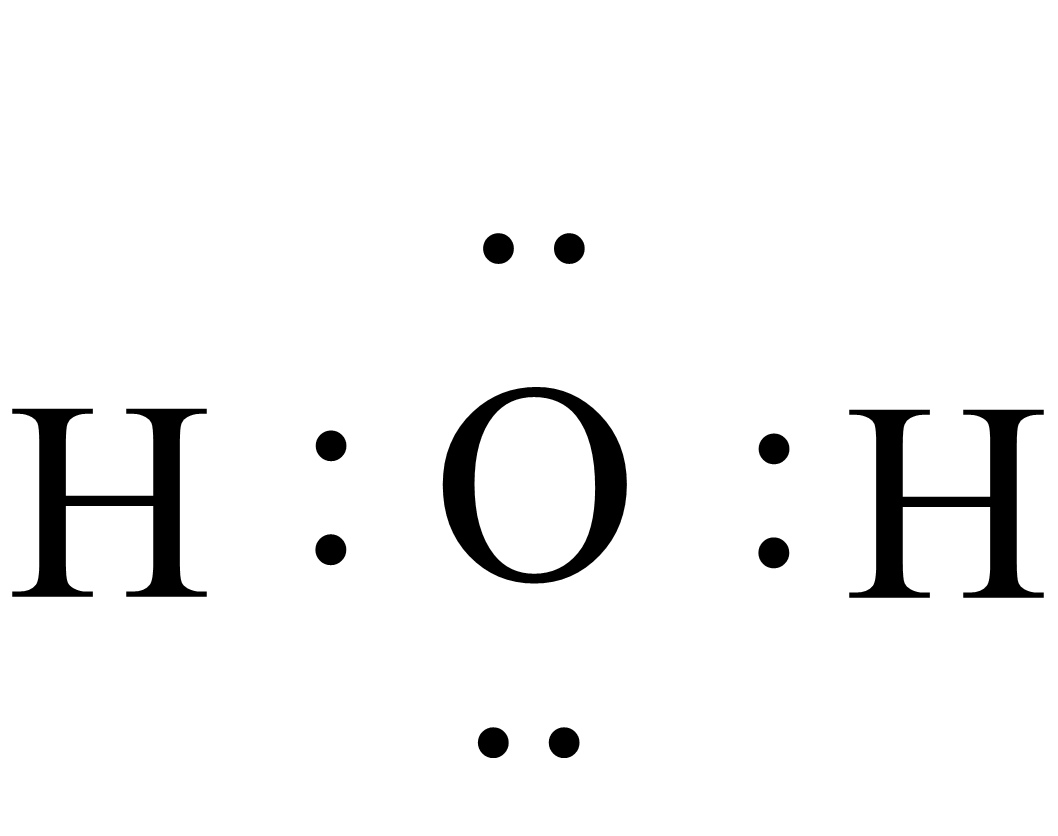
ii.Lewis dot structure of carbon tetrachloride($CC{l_4}$) is:
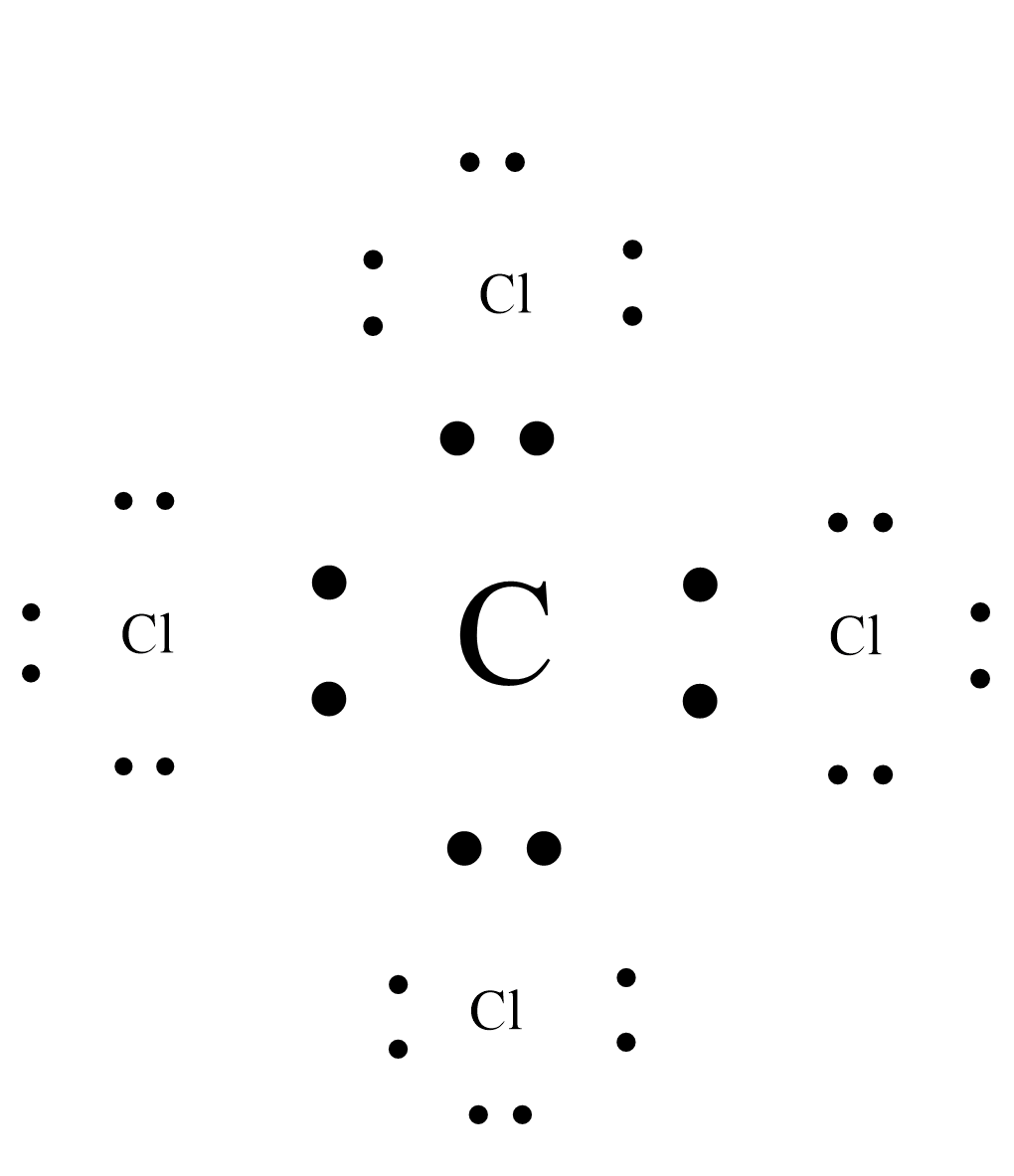
iii.Lewis dot structure of dihydrogen( ${H_2}$) is:
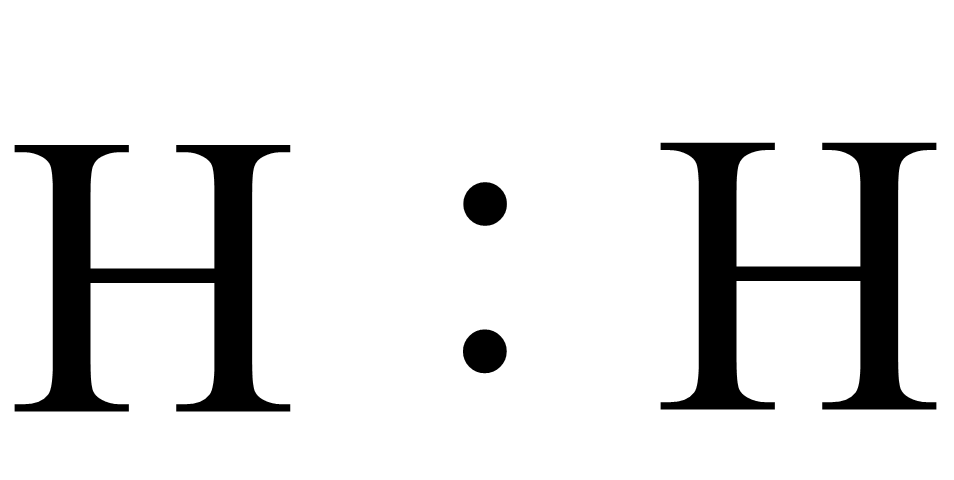
Note:
Some limitations of the lewis dot structure are discussed below:
It fails to explain the cause of covalent bond formation.
It is not able to explain the geometry of molecules containing a covalent bond.
It does not tell about the exchange of energy during the formation of a covalent bond.
It does not give any idea about the formation of the electron-deficient compound such as $B{F_3},{\text{ }}AlC{l_3}$, etc which violates the octet rule.
It also does not explain the formation of electron excess compounds such as $PC{l_5},{\text{ }}S{F_6}$, etc.
It does not give any idea about the attractive force between the constituent atoms of a molecule.
Complete step by step answer:
The steps needed to be followed to write the lewis dot structure:
Calculate the number of valence electrons of an atom present.
If the given species is an anion then add the number of electrons equal to a unit of negative charge and if the given species is cation then subtract the number of electrons equal to a unit of a positive charge.
Select the central atom and draw the skeleton structure with intelligence guesses.
Remember that hydrogen and fluorine usually occupy the terminal position.
Put the shared pair of electrons between two atoms to represent the single bond and put the remaining electrons to represent multiple bonds or show them as a lone pair.
The lewis dot structure of the given compound is written as:
i.Lewis dot structure of water (${H_2}O$) is:
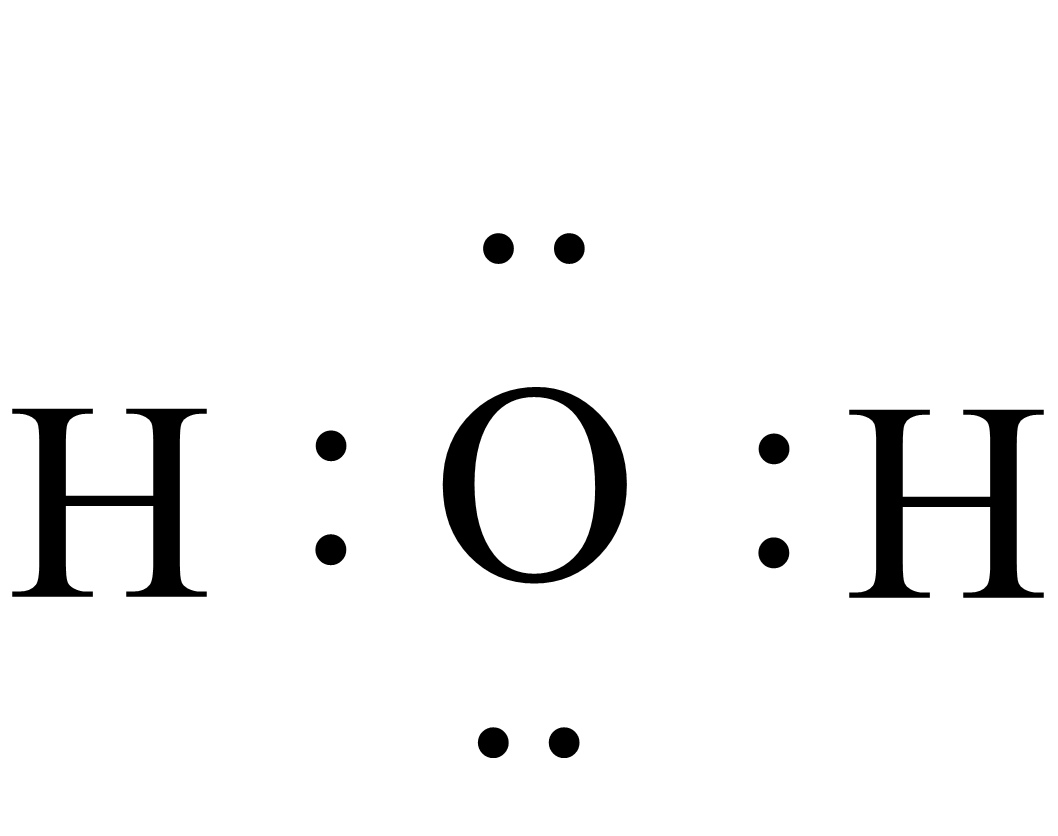
ii.Lewis dot structure of carbon tetrachloride($CC{l_4}$) is:

iii.Lewis dot structure of dihydrogen( ${H_2}$) is:

Note:
Some limitations of the lewis dot structure are discussed below:
It fails to explain the cause of covalent bond formation.
It is not able to explain the geometry of molecules containing a covalent bond.
It does not tell about the exchange of energy during the formation of a covalent bond.
It does not give any idea about the formation of the electron-deficient compound such as $B{F_3},{\text{ }}AlC{l_3}$, etc which violates the octet rule.
It also does not explain the formation of electron excess compounds such as $PC{l_5},{\text{ }}S{F_6}$, etc.
It does not give any idea about the attractive force between the constituent atoms of a molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




