
Write the Lewis structure of the nitrite ion, $N{{O}_{2}}^{-}$ .
Answer
542.1k+ views
Hint: For drawing the Lewis structure of any molecule we have to follow some of the well dignified steps. Also, we can straight draw the structure before considering other factors such as bond angle but if we consider that would be better than before.
Complete step-by-step answer:Let us directly see the steps to be followed to draw Lewis structure of nitrite ion;
Firstly, we need to find the total number of valence electrons of nitrogen and oxygen and also the charge on the anion.
Then we need to know or calculate the total electron pairs.
We need to select the central atom out of nitrogen and oxygen.
Next, we need to put the lone pairs on the atoms.
Finally, we need to check the stability of the Lewis structure and minimize the charge on the atom by converting the pair of electrons into bonds between the atoms.
Here,
Total valence electrons coming from nitrogen side = 5
Total valence electrons coming from oxygen side = $6\times 2=12$
The charge present on the nitrite ion = -1; so, one electron will be added to the total electrons.
Thus,
Total valence electrons = 5 + 12 + 1 = 18; which would be distributed as, $\sigma $ bonds + $\Pi $ bonds + lone pairs at valence shells
After knowing this, we must follow other steps to reach the final structure of nitrite ion as follows;
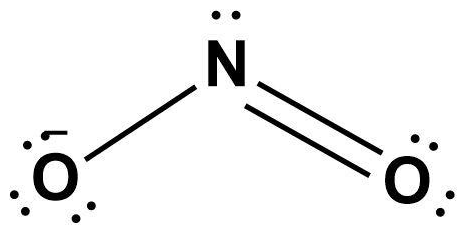
Note: Do note that the negative charge on the molecule must be considered compulsorily along with total valence electrons of nitrogen and oxygen atoms while drawing the Lewis structure.
Do note that the distribution of electrons influences the geometry of the ion so the structure has some bond angle.
Complete step-by-step answer:Let us directly see the steps to be followed to draw Lewis structure of nitrite ion;
Firstly, we need to find the total number of valence electrons of nitrogen and oxygen and also the charge on the anion.
Then we need to know or calculate the total electron pairs.
We need to select the central atom out of nitrogen and oxygen.
Next, we need to put the lone pairs on the atoms.
Finally, we need to check the stability of the Lewis structure and minimize the charge on the atom by converting the pair of electrons into bonds between the atoms.
Here,
Total valence electrons coming from nitrogen side = 5
Total valence electrons coming from oxygen side = $6\times 2=12$
The charge present on the nitrite ion = -1; so, one electron will be added to the total electrons.
Thus,
Total valence electrons = 5 + 12 + 1 = 18; which would be distributed as, $\sigma $ bonds + $\Pi $ bonds + lone pairs at valence shells
After knowing this, we must follow other steps to reach the final structure of nitrite ion as follows;

Note: Do note that the negative charge on the molecule must be considered compulsorily along with total valence electrons of nitrogen and oxygen atoms while drawing the Lewis structure.
Do note that the distribution of electrons influences the geometry of the ion so the structure has some bond angle.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




