
Write the mechanism of hydration of ethylene to ethyl alcohol.
Answer
573.3k+ views
Hint: To answer this question, you should recall the concept of hydration of alkenes to alcohols. This process involves electrophilic addition reaction for ethylene. For conversion to ethyl alcohol, Ethene is mixed with steam and passed over a catalyst consisting of solid silicon dioxide coated with phosphoric(V) acid. The temperature used is \[300{^\circ C}\] and the pressure is about 60 to 70 atmospheres.
Complete Step by step solution:
Alkenes refer to a group of unsaturated hydrocarbons that is one molecule of alkene containing at least one double bond. Due to the presence of pi electrons, they show additional reactions which involve an electrophile attacking the carbon-carbon double bond to form the addition products. These reactions are known as electrophilic addition reactions of alkenes.
The overall mechanism of this process can be summarized as:
Step 1: The hydrogen atoms of phosphoric(V) acid have a partial positive charge due to large disparity in electronegativities due to which these hydrogens are strongly attracted to the carbon-carbon double bond. The pi part of the bond breaks and the electrons in it move down to make a new bond with the hydrogen atom. The reaction can be represented as:
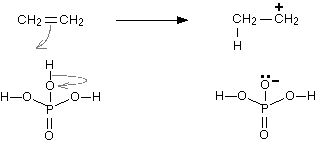
Step 2: This newly formed carbocation reacts with one of the lone pairs on a water molecule. The reaction can be represented as:
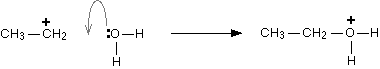
Step 3: Finally, one of the hydrogens on the oxygen is removed by reaction with the dihydrogen phosphate(V) ion, \[{H_2}P{O_4}^ - \], formed in the first step. The reaction can be represented as:
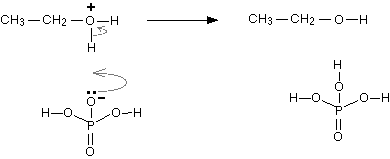
Note: You should know that alcohols are amphoteric. The lone pair of electrons on oxygen makes the \[{\text{-OH}}\] group a weak base. In this molecule, the oxygen can donate two electrons to an electron-deficient proton. This means that in the presence of a strong acid, \[{\text{R-OH}}\] acts as a base and protonated into the very acidic alkyloxonium ion. This weakly basic nature of alcohols is essential for its dehydration reaction with an acid to form alkenes. The temperature range for each substituted alcohol is different:\[1^\circ :{\text{ }}170^\circ {\text{ }} - {\text{ }}180{^\circ C},{\text{ }}2^\circ :100^\circ -{\text{ }}140{ ^\circ C},{\text{ }}3^\circ :{\text{ }}25^\circ -{\text{ }}80{^\circ C}\]
Complete Step by step solution:
Alkenes refer to a group of unsaturated hydrocarbons that is one molecule of alkene containing at least one double bond. Due to the presence of pi electrons, they show additional reactions which involve an electrophile attacking the carbon-carbon double bond to form the addition products. These reactions are known as electrophilic addition reactions of alkenes.
The overall mechanism of this process can be summarized as:
Step 1: The hydrogen atoms of phosphoric(V) acid have a partial positive charge due to large disparity in electronegativities due to which these hydrogens are strongly attracted to the carbon-carbon double bond. The pi part of the bond breaks and the electrons in it move down to make a new bond with the hydrogen atom. The reaction can be represented as:
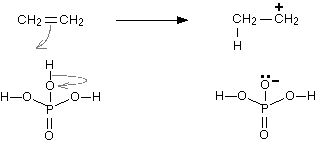
Step 2: This newly formed carbocation reacts with one of the lone pairs on a water molecule. The reaction can be represented as:
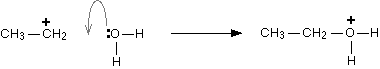
Step 3: Finally, one of the hydrogens on the oxygen is removed by reaction with the dihydrogen phosphate(V) ion, \[{H_2}P{O_4}^ - \], formed in the first step. The reaction can be represented as:

Note: You should know that alcohols are amphoteric. The lone pair of electrons on oxygen makes the \[{\text{-OH}}\] group a weak base. In this molecule, the oxygen can donate two electrons to an electron-deficient proton. This means that in the presence of a strong acid, \[{\text{R-OH}}\] acts as a base and protonated into the very acidic alkyloxonium ion. This weakly basic nature of alcohols is essential for its dehydration reaction with an acid to form alkenes. The temperature range for each substituted alcohol is different:\[1^\circ :{\text{ }}170^\circ {\text{ }} - {\text{ }}180{^\circ C},{\text{ }}2^\circ :100^\circ -{\text{ }}140{ ^\circ C},{\text{ }}3^\circ :{\text{ }}25^\circ -{\text{ }}80{^\circ C}\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




