
How do you write the orbital diagram for silver?
Answer
547.8k+ views
Hint: According to Dalton’s theory the atom is the most basic form of matter but the atom contains various subatomic particles like the electrons and protons. The atomic structure of the atom depends on the electronic configuration in which the electrons are arranged inside the atoms. This electronic configuration is responsible for various properties that the atom shows.
Complete step by step answer:
The atom consists of electrons which are present inside the atoms in specific orbits or shells. These electrons are present in the shells according to the energy. The distribution of the electron is similar to how the planets move in our solar system. The electrons are placed in shells according to the energy. These shells are also given naming convention which helps in the description of any electron that is present in the atomic shells.
All the electrons in the atom have some quantum numbers which are used to describe its location and thus also help in inferring the properties that the element is going to show.
The quantum numbers are namely the principle quantum number, the azimuthal quantum number, magnetic quantum number and the spin quantum number.
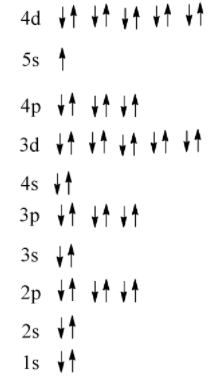
From the periodic table we know that the at atomic number of silver is 47 so it will have the electronic configuration of $[Kr]\,5{s^1}4{d^{10}}$ this happens in the place of the expected $5{s^2}4{d^9}$ due to Hund’s rule which states that full filled and half-filled electronic configurations are more stable .
Note: Azimuthal quantum number shows the subshells and determines the shape of the orbital. It can also be used to determine the number of angular nodes.
The magnetic quantum number describes the energy levels in a subshells and is responsible for the magnetic properties.
Complete step by step answer:
The atom consists of electrons which are present inside the atoms in specific orbits or shells. These electrons are present in the shells according to the energy. The distribution of the electron is similar to how the planets move in our solar system. The electrons are placed in shells according to the energy. These shells are also given naming convention which helps in the description of any electron that is present in the atomic shells.
All the electrons in the atom have some quantum numbers which are used to describe its location and thus also help in inferring the properties that the element is going to show.
The quantum numbers are namely the principle quantum number, the azimuthal quantum number, magnetic quantum number and the spin quantum number.
From the periodic table we know that the at atomic number of silver is 47 so it will have the electronic configuration of $[Kr]\,5{s^1}4{d^{10}}$ this happens in the place of the expected $5{s^2}4{d^9}$ due to Hund’s rule which states that full filled and half-filled electronic configurations are more stable .

Note: Azimuthal quantum number shows the subshells and determines the shape of the orbital. It can also be used to determine the number of angular nodes.
The magnetic quantum number describes the energy levels in a subshells and is responsible for the magnetic properties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




