
Write the preparation of benzoic acid from the following:
(a) Styrene
(b) Benzamide
(c) Dry ice
Answer
529.5k+ views
Hint: To solve this question, we first need to know what benzoic acid, styrene, benzamide, and dry ice.
Benzoic acid: A colorless and crystalline derivative of benzene having formula ${{C}_{6}}{{H}_{5}}COOH$
Styrene: A colorless and oily derivative of benzene having formula ${{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}$.
Benzamide: A white and solid derivative of benzoic acid having formula ${{C}_{6}}{{H}_{5}}C(O)N{{H}_{2}}$.
Dry ice: A cooling agent which is the solid form of the gas carbon dioxide $C{{O}_{2}}$.
Complete answer:
Let us look at the reactions involving the preparation of benzoic acid from styrene, benzamide, and dry ice.
a) Using styrene (phenylethane) to prepare benzoic acid.
While converting styrene to benzoic acid, an oxidizing agent is used.
Either potassium permanganate or potassium dichromate, in the presence of sulfuric acid, acts as a strong oxidizing agent and converts styrene to benzoic acid.
Styrene is first converted to potassium benzoate and then acidified to give benzoic acid.
The reaction proceeds as follows:
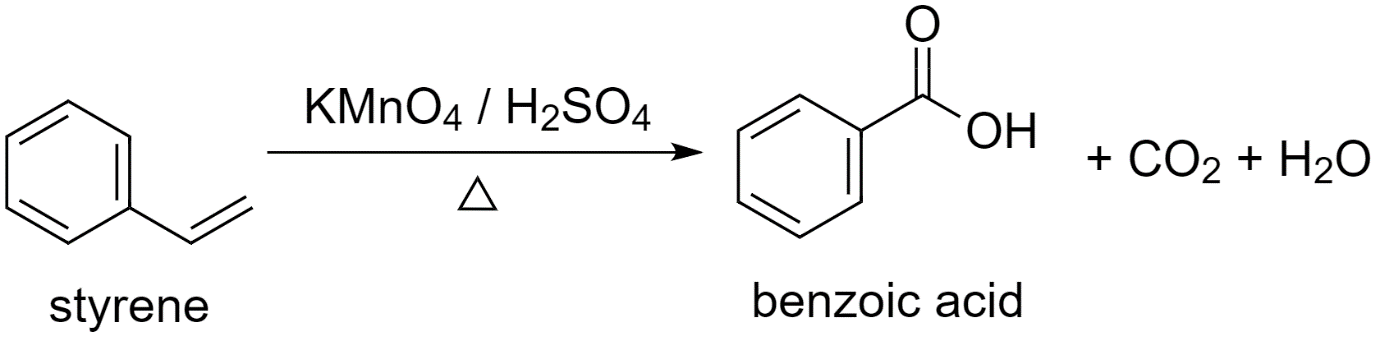
Along with benzoic acid, carbon dioxide $C{{O}_{2}}$ gas is released and a water ${{H}_{2}}O$ molecule is produced as by-products.
b) Using benzamide to prepare benzoic acid.
In the presence of an acid like dilute hydrochloric acid, the hydrolysis of benzamide with sodium hydroxide (NaOH) forms benzoic acid.
Benzamide is first converted to sodium benzoate which is further acidified to benzoic acid.
The reaction proceeds as follows:
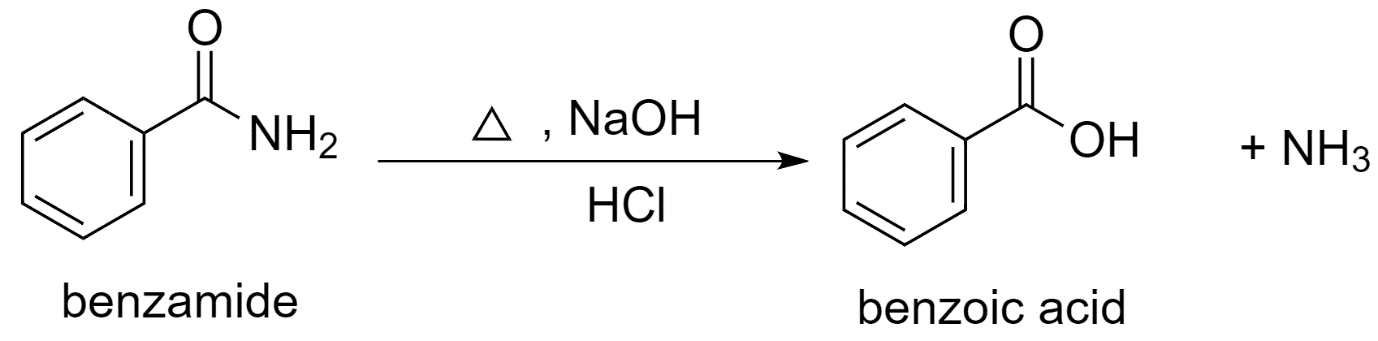
Along with benzoic acid, ammonia $N{{H}_{3}}$ is released as the by-product.
c) Using dry ice to prepare benzoic acid.
When dry ice or solid carbon dioxide $C{{O}_{2}}$ is added to a solution of phenyl magnesium iodide in dry ether, an adduct is formed. The adduct formed is a magnesium salt of carboxylic acid.
When this adduct is hydrolyzed in the presence of an acid, it gives benzoic acid.
The reaction proceeds as follows:
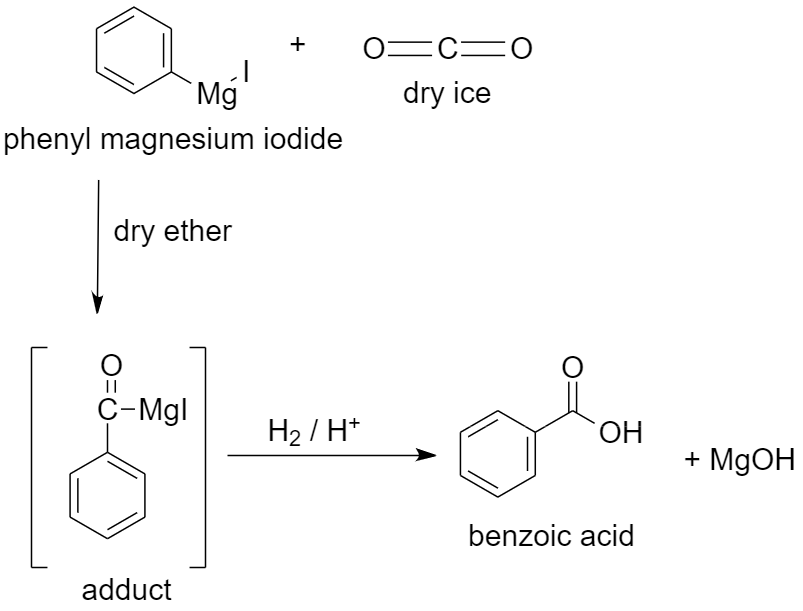
Along with benzoic acid, magnesium hydroxide (MgOH) is formed as a by-product.
Note:
It should be noted that the gas released from dry ice can increase the levels of carbon dioxide $C{{O}_{2}}$ in the blood and can cause hypercapnia.
Also, styrene is considered to be carcinogenic and toxic, especially when in contact with skin or eyes.
Benzoic acid on the other hand is produced naturally in many plants and animal species.
Benzoic acid: A colorless and crystalline derivative of benzene having formula ${{C}_{6}}{{H}_{5}}COOH$
Styrene: A colorless and oily derivative of benzene having formula ${{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}$.
Benzamide: A white and solid derivative of benzoic acid having formula ${{C}_{6}}{{H}_{5}}C(O)N{{H}_{2}}$.
Dry ice: A cooling agent which is the solid form of the gas carbon dioxide $C{{O}_{2}}$.
Complete answer:
Let us look at the reactions involving the preparation of benzoic acid from styrene, benzamide, and dry ice.
a) Using styrene (phenylethane) to prepare benzoic acid.
While converting styrene to benzoic acid, an oxidizing agent is used.
Either potassium permanganate or potassium dichromate, in the presence of sulfuric acid, acts as a strong oxidizing agent and converts styrene to benzoic acid.
Styrene is first converted to potassium benzoate and then acidified to give benzoic acid.
The reaction proceeds as follows:

Along with benzoic acid, carbon dioxide $C{{O}_{2}}$ gas is released and a water ${{H}_{2}}O$ molecule is produced as by-products.
b) Using benzamide to prepare benzoic acid.
In the presence of an acid like dilute hydrochloric acid, the hydrolysis of benzamide with sodium hydroxide (NaOH) forms benzoic acid.
Benzamide is first converted to sodium benzoate which is further acidified to benzoic acid.
The reaction proceeds as follows:

Along with benzoic acid, ammonia $N{{H}_{3}}$ is released as the by-product.
c) Using dry ice to prepare benzoic acid.
When dry ice or solid carbon dioxide $C{{O}_{2}}$ is added to a solution of phenyl magnesium iodide in dry ether, an adduct is formed. The adduct formed is a magnesium salt of carboxylic acid.
When this adduct is hydrolyzed in the presence of an acid, it gives benzoic acid.
The reaction proceeds as follows:

Along with benzoic acid, magnesium hydroxide (MgOH) is formed as a by-product.
Note:
It should be noted that the gas released from dry ice can increase the levels of carbon dioxide $C{{O}_{2}}$ in the blood and can cause hypercapnia.
Also, styrene is considered to be carcinogenic and toxic, especially when in contact with skin or eyes.
Benzoic acid on the other hand is produced naturally in many plants and animal species.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




