
Write the reactions involved in the conversion of propylene dichloride to isopropyl bromide.
Answer
571.8k+ views
Hint: When propylene or any unsaturated compound needs to be converted to saturate (Single bonded), it involved Markonikov’s rule of addition of hydrogen atom. Free radical addition reactions do not obey Markonikov’s rule since the selectivity of the mechanisms of these reactions are not predicted by Markonikov’s rule. These reactions are generally referred to as Anti-Markovnikov's additional reactions.
Complete step by step answer:
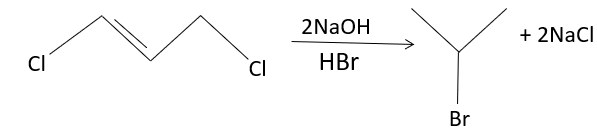
The reaction involved in the conversion of propylene dichloride to isopropyl bromide involves Markonikov’s rule. Here, the propylene is converted to the propane group, by the addition of the hydrogen to the carbon-containing more hydrogen, and the halide is added to a carbon-containing least number of the hydrogen atom. Here, the sodium hydroxide is used to remove the chloride and the hydrogen bromide is used to hydrogenate, and also induce bromine atoms to the compound. When propylene dichloride is treated with Hydrogen bromide, the hydrogen bromide dissociates into hydrogen ion and bromide ion involves the formation of the carbocation, to which the electro-negative bromine attaches to form 2-Bromo propane or isopropyl bromide and sodium chloride.
Note: The Russian chemist Vladimir Vasilyevich Markonikov’s first formulated this rule in 1865. When alkenes are treated with borane $(BH_3)$ in the presence of hydrogen peroxide or sodium hydroxide, alcohol is obtained as the final product. In this electrophilic addition reaction, the boron atom acts as an electrophile. This reaction does not obey Markonikov’s rule and can, therefore, be classified as an anti-Markovnikov's reaction.
Complete step by step answer:
The reaction involved in the conversion of propylene dichloride to isopropyl bromide involves Markonikov’s rule. Here, the propylene is converted to the propane group, by the addition of the hydrogen to the carbon-containing more hydrogen, and the halide is added to a carbon-containing least number of the hydrogen atom. Here, the sodium hydroxide is used to remove the chloride and the hydrogen bromide is used to hydrogenate, and also induce bromine atoms to the compound. When propylene dichloride is treated with Hydrogen bromide, the hydrogen bromide dissociates into hydrogen ion and bromide ion involves the formation of the carbocation, to which the electro-negative bromine attaches to form 2-Bromo propane or isopropyl bromide and sodium chloride.

Note: The Russian chemist Vladimir Vasilyevich Markonikov’s first formulated this rule in 1865. When alkenes are treated with borane $(BH_3)$ in the presence of hydrogen peroxide or sodium hydroxide, alcohol is obtained as the final product. In this electrophilic addition reaction, the boron atom acts as an electrophile. This reaction does not obey Markonikov’s rule and can, therefore, be classified as an anti-Markovnikov's reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




