
Write the structure of isomeric alkanes of molecular formula $ {C_6}{H_{10}} $ that yield $ {\text{2 - methylpentane}} $ on catalytic hydrogenation.
Answer
527.4k+ views
Hint: Here, we have been asked to write the isomeric alkanes of molecular formula $ {C_6}{H_{10}} $ for this first we have to understand the meaning of isomerism or isomers of some compound. So that we will be able to find the answer to this question. Also we have to study the structure forming characteristics of the chemical formula of the compound by the norms of IUPAC. Here, the yield of $ {\text{2 - methylpentane}} $ is obtained on catalytic hydrogenation so we have to form a reaction for the conditions given here. In this way we can be able to answer this question.
Complete answer:
let us first form a chemical reaction according to the given conditions as $ {C_6}{H_{10}} $ on catalytic hydrogenation gives $ {\text{2 - methylpentane}} $ as represented in the chemical reaction below:
$ {C_6}{H_{10}}\xrightarrow{{{H_2}({\text{catalytic hydrogenation}})}}{\text{2 - methylpentane}} $
The structural formula for the product yield $ {\text{2 - methylpentane}} $ is given by:
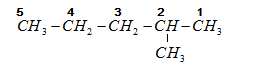
The numbers are given on carbons in the formula above.
Now, the structural formula for the isomeric alkynes (having triple bond) of $ {\text{2 - methylpentane}} $ we have to understand the meaning of isomers as “Isomers are compounds that contain exactly the same number of atoms or having exactly the same empirical formula, but different structural formula from each other by the way in which the atoms are arranged.”
So, the isomers of $ {\text{2 - methylpentane}} $ are:
(a)
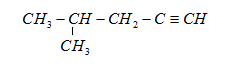
(b)
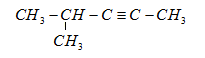
In these structures, we see that the structural formula is different but the empirical formula or the number of carbons and hydrogens in this are the same.
These are the isomeric alkynes of the yield obtained from hydrogenation of $ {C_6}{H_{10}} $.
Note:
Here, the isomers are the compounds having the same empirical formula but different structural formula as we have observed above. We have to be careful while placing the carbon and hydrogen in the structural formula because of the valence they possess.
Complete answer:
let us first form a chemical reaction according to the given conditions as $ {C_6}{H_{10}} $ on catalytic hydrogenation gives $ {\text{2 - methylpentane}} $ as represented in the chemical reaction below:
$ {C_6}{H_{10}}\xrightarrow{{{H_2}({\text{catalytic hydrogenation}})}}{\text{2 - methylpentane}} $
The structural formula for the product yield $ {\text{2 - methylpentane}} $ is given by:
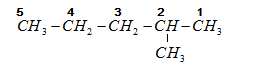
The numbers are given on carbons in the formula above.
Now, the structural formula for the isomeric alkynes (having triple bond) of $ {\text{2 - methylpentane}} $ we have to understand the meaning of isomers as “Isomers are compounds that contain exactly the same number of atoms or having exactly the same empirical formula, but different structural formula from each other by the way in which the atoms are arranged.”
So, the isomers of $ {\text{2 - methylpentane}} $ are:
(a)
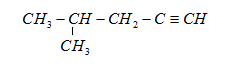
(b)
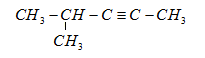
In these structures, we see that the structural formula is different but the empirical formula or the number of carbons and hydrogens in this are the same.
These are the isomeric alkynes of the yield obtained from hydrogenation of $ {C_6}{H_{10}} $.
Note:
Here, the isomers are the compounds having the same empirical formula but different structural formula as we have observed above. We have to be careful while placing the carbon and hydrogen in the structural formula because of the valence they possess.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




