
Write the structure of the p-methylbenzaldehyde molecule.
Answer
579.6k+ views
Hint: The name p-methylbenzaldehyde suggests that the methyl group is attached to the para position of the aldehyde group. Identify the methyl group, aldehyde group, para position and attach them to the benzene ring.
Complete answer:
Write the structure of p-methylbenzaldehyde molecule as follows:
The p-methylbenzaldehyde suggests that to a benzene ring, an aldehyde group is attached. A methyl group is attached at a para position.
The aldehyde group is $ - {\text{CHO}}$. The methyl group is $ - {\text{C}}{{\text{H}}_3}$.
Thus, the structure of p-methylbenzaldehyde molecule is as follows:
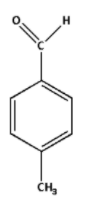
Additional Information: para or ‘p’ describes a molecule in which the substituents are at 1 and 4 positions on an aromatic compound. Similarly, ortho or o describes a molecule in which the substituents are at 2 and 6 positions on an aromatic compound, and meta or m describes a molecule in which the substituents are at 3 and 5 positions on an aromatic compound.
Note: p-methylbenzaldehyde is also known as 4-methylbenzaldehyde or p-tolualdehyde.
The methyl group is attached at carbon number 4 of the benzene ring. Thus, the name is 4- methylbenzaldehyde.
The compound in which a methyl group is attached to a benzene ring is known as toluene. The methyl group is attached to the para position of the benzene ring to which an aldehyde group is attached. Thus, the name is p-tolualdehyde.
p-methylbenzaldehyde is an aromatic aldehyde and a colourless liquid. It smells like cherry, similar to benzaldehyde.
It can be prepared by the Friedel Craft formylation of toluene with hydrogen chloride and carbon monoxide.
Complete answer:
Write the structure of p-methylbenzaldehyde molecule as follows:
The p-methylbenzaldehyde suggests that to a benzene ring, an aldehyde group is attached. A methyl group is attached at a para position.
The aldehyde group is $ - {\text{CHO}}$. The methyl group is $ - {\text{C}}{{\text{H}}_3}$.
Thus, the structure of p-methylbenzaldehyde molecule is as follows:

Additional Information: para or ‘p’ describes a molecule in which the substituents are at 1 and 4 positions on an aromatic compound. Similarly, ortho or o describes a molecule in which the substituents are at 2 and 6 positions on an aromatic compound, and meta or m describes a molecule in which the substituents are at 3 and 5 positions on an aromatic compound.
Note: p-methylbenzaldehyde is also known as 4-methylbenzaldehyde or p-tolualdehyde.
The methyl group is attached at carbon number 4 of the benzene ring. Thus, the name is 4- methylbenzaldehyde.
The compound in which a methyl group is attached to a benzene ring is known as toluene. The methyl group is attached to the para position of the benzene ring to which an aldehyde group is attached. Thus, the name is p-tolualdehyde.
p-methylbenzaldehyde is an aromatic aldehyde and a colourless liquid. It smells like cherry, similar to benzaldehyde.
It can be prepared by the Friedel Craft formylation of toluene with hydrogen chloride and carbon monoxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




