
How do you write/draw the Lewis structure for N?
Answer
564.9k+ views
Hint Lewis dot structures also known as electron dot structures. Lewis dot structures represent the valence electrons of the atoms present within the molecule. By Lewis dot structures we can visualize the bonding electrons and lone pair of electrons present in the molecule.
Complete step by step answer:
- In the question it is asked to draw the Lewis structure for N (nitrogen atom).
- Nitrogen belongs to group 15 and is present in the p-block of the periodic table.
- The atomic number of nitrogen is 7.
- Means nitrogen has 7 electrons in its electronic configuration.
- The electronic configuration of the nitrogen atom is $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$ .
- From the above electronic configuration we can say that the nitrogen has 5 five electrons in 2s and 2p orbitals and the number of valence electrons are five in nitrogen.
- The Lewis dot structure of nitrogen atom is as follows.
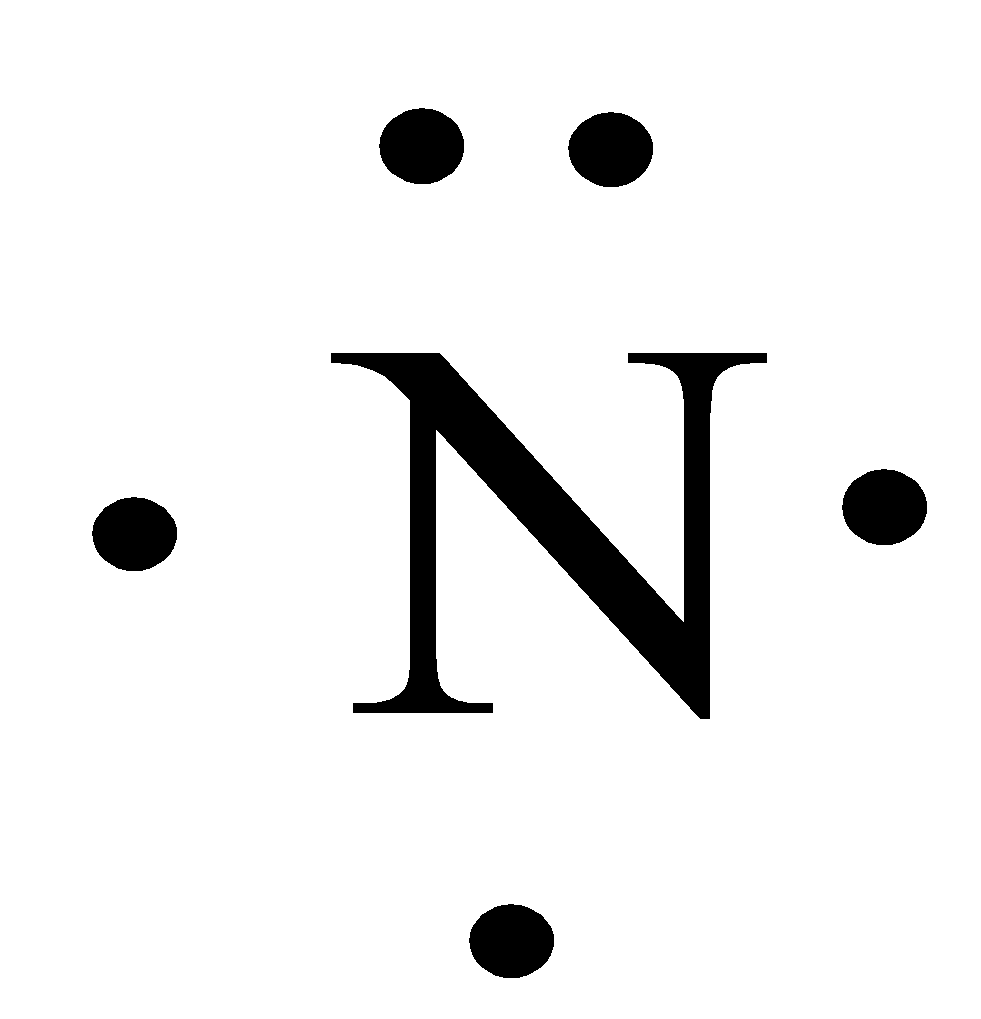
- From the above Lewis dot structure of nitrogen we can say that nitrogen has only one lone pair of electrons and three single electrons around the nitrogen atom.
Note: Without knowing the electronic configuration of the atom we cannot draw the Lewis dot structure of the atoms or elements. The three single electrons present around the nitrogen makes the nitrogen atom to form three bonds with other atoms. In all the compounds which have nitrogen atoms in its composition, nitrogen will have three bonds with other atoms.
Complete step by step answer:
- In the question it is asked to draw the Lewis structure for N (nitrogen atom).
- Nitrogen belongs to group 15 and is present in the p-block of the periodic table.
- The atomic number of nitrogen is 7.
- Means nitrogen has 7 electrons in its electronic configuration.
- The electronic configuration of the nitrogen atom is $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$ .
- From the above electronic configuration we can say that the nitrogen has 5 five electrons in 2s and 2p orbitals and the number of valence electrons are five in nitrogen.
- The Lewis dot structure of nitrogen atom is as follows.

- From the above Lewis dot structure of nitrogen we can say that nitrogen has only one lone pair of electrons and three single electrons around the nitrogen atom.
Note: Without knowing the electronic configuration of the atom we cannot draw the Lewis dot structure of the atoms or elements. The three single electrons present around the nitrogen makes the nitrogen atom to form three bonds with other atoms. In all the compounds which have nitrogen atoms in its composition, nitrogen will have three bonds with other atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




