
Zone refining is based on the principle that:
(A) impurities of low boiling metals can be separated by distillation
(B) impurities are more soluble in molten metal than in solid metal
(C) different component of a mixture are differently adsorbed on an adsorbent
(D) vapours of volatile compounds can be decomposed in pure metal
Answer
526k+ views
Hint: Revise the concepts of metallurgy. Zone refining is a process used to separate pure metal from the impurities. Recollect what happens in the process of zone refining. Read all the options and tick the most suitable answer.
Complete step by step answer:
- Zone refining is a metallurgical process used to obtain pure metal.
- In the Zone refining process, pure metal is separated from its impurities.
- In this process, a narrow rod of metal is placed inside a heater. The heater moves up and down across the rod, each time melting just one particular cross-sectional area of the rod known as zone.
- A schematic representation of the Zone refining process is shown below.
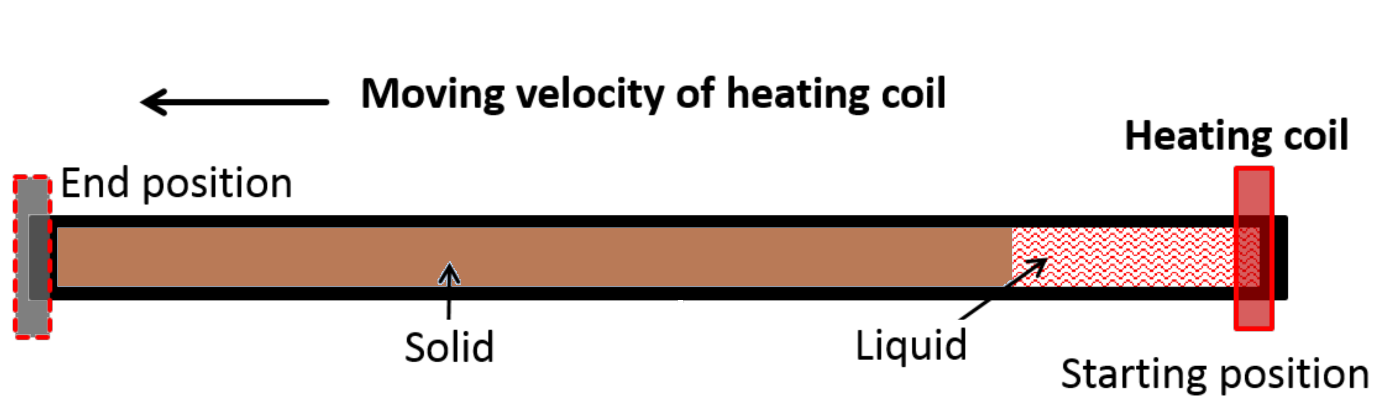
- In the zone refining process, impurities present in the metal dissolve readily in the molten metal and this molten metal containing impurities travels from start position to end position.
- At the end of the zone refining process, all the impurities settle at the bottom part of the rod and this part of the rod containing maximum impurities is cut and separated from the pure metal rod.
- Zone refining process works on the principle that impurities are more soluble in molten metal than solid metal.
So, the correct answer is “Option B”.
Note: Remember zone refining process is based on the principle of relative solubilities of impurities in molten metal. Gallium and Germanium are examples of metals purified by zone refining method. Remember in the zone refining process, a heating coil moves along the metal rod melting it at small zones and this process is repeated many times to obtain all impurities at the end of the rod which are then cut and separated from the pure metal.
Complete step by step answer:
- Zone refining is a metallurgical process used to obtain pure metal.
- In the Zone refining process, pure metal is separated from its impurities.
- In this process, a narrow rod of metal is placed inside a heater. The heater moves up and down across the rod, each time melting just one particular cross-sectional area of the rod known as zone.
- A schematic representation of the Zone refining process is shown below.

- In the zone refining process, impurities present in the metal dissolve readily in the molten metal and this molten metal containing impurities travels from start position to end position.
- At the end of the zone refining process, all the impurities settle at the bottom part of the rod and this part of the rod containing maximum impurities is cut and separated from the pure metal rod.
- Zone refining process works on the principle that impurities are more soluble in molten metal than solid metal.
So, the correct answer is “Option B”.
Note: Remember zone refining process is based on the principle of relative solubilities of impurities in molten metal. Gallium and Germanium are examples of metals purified by zone refining method. Remember in the zone refining process, a heating coil moves along the metal rod melting it at small zones and this process is repeated many times to obtain all impurities at the end of the rod which are then cut and separated from the pure metal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




