Class 11 Biology Chapter 9 Summary Notes PDF Download
FAQs on Biomolecules Class 11 Biology Chapter 9 CBSE Notes - 2025-26
1. What key topics are summarised in the Class 11 Biomolecules revision notes?
These notes provide a comprehensive summary of Chapter 9, focusing on core concepts essential for the 2025-26 CBSE syllabus. Key topics covered include:
- Analysis of chemical composition in living tissues.
- An overview of primary and secondary metabolites.
- Detailed explanations of biomacromolecules like proteins, polysaccharides, and nucleic acids.
- The structure and function of lipids.
- The nature of enzyme action, including factors affecting it and their classification.
2. How are these revision notes structured for effective and quick learning?
The notes are designed for efficient revision. They break down complex topics into simple, easy-to-digest points. Key information is highlighted, and concepts are explained with clear summaries and supporting diagrams. This structure helps you quickly review and recall important definitions, structures, and functions, making them ideal for last-minute preparation.
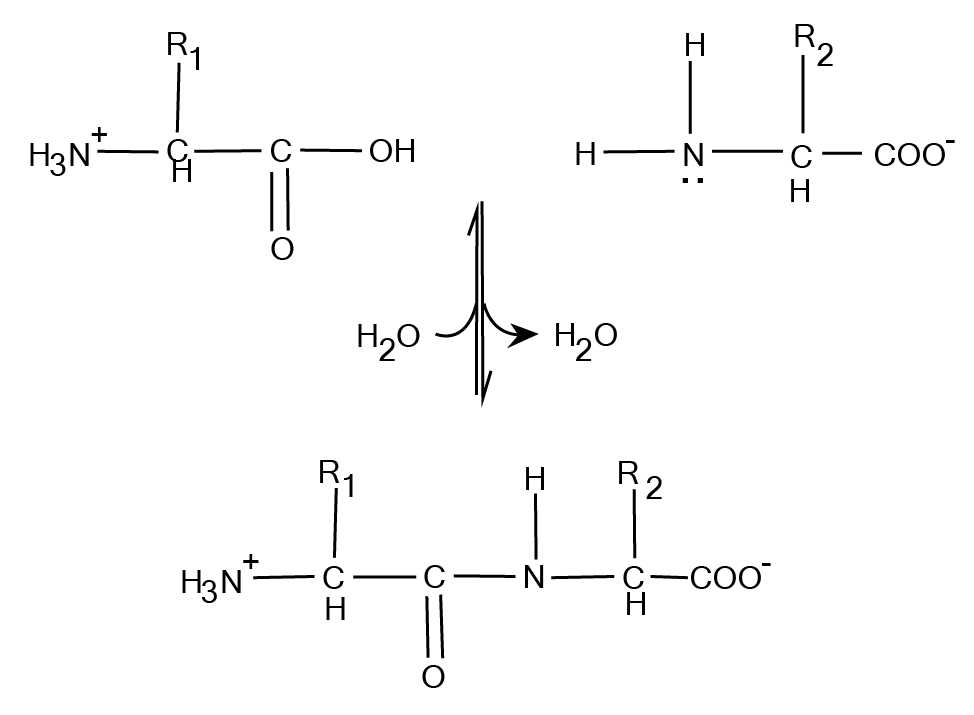
3. What is a quick summary of protein structure as explained in these notes?
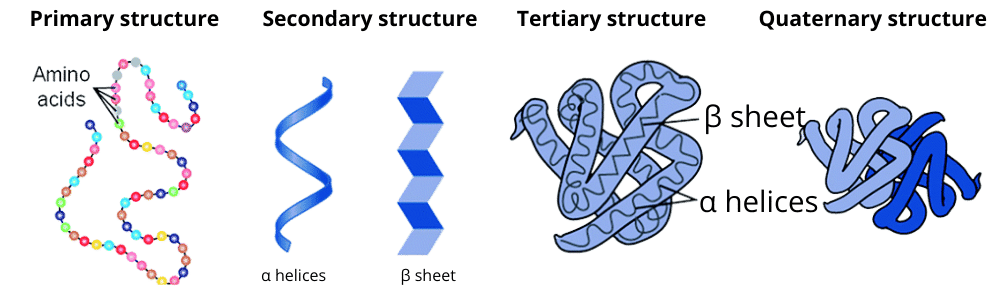
The notes summarise the four levels of protein organisation:
- Primary Structure: The linear sequence of amino acids in a polypeptide chain.
- Secondary Structure: The local folding of the polypeptide into structures like the α-helix and β-pleated sheet, stabilised by hydrogen bonds.
- Tertiary Structure: The overall three-dimensional shape of a single polypeptide chain.
- Quaternary Structure: The arrangement of multiple polypeptide chains to form a functional protein, such as haemoglobin.
4. How do the notes explain the difference between primary and secondary metabolites for quick recall?
The revision notes clarify this by summarising their key roles. Primary metabolites, such as amino acids and sugars, are described as having identifiable functions in normal physiological processes and are essential for growth and development. In contrast, secondary metabolites (e.g., alkaloids, essential oils) are explained as compounds found mainly in plants and microbes, whose direct role in the host is often unclear but they have significant ecological or human utility.
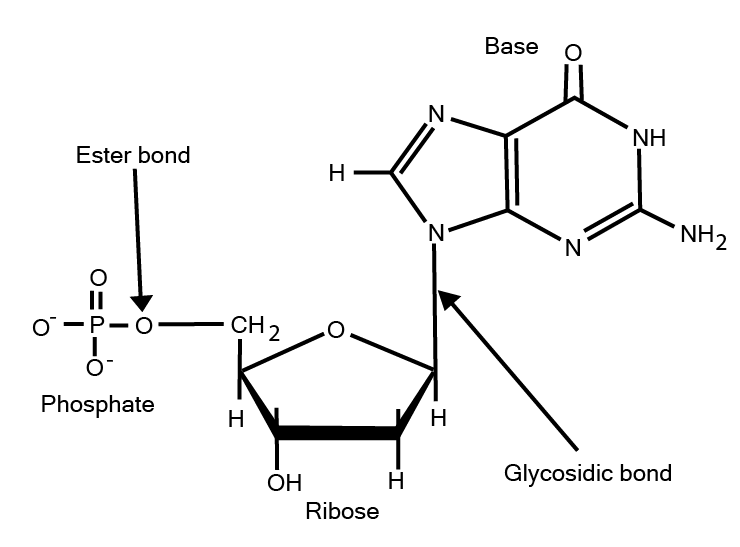
5. How can I use these notes to quickly revise the key differences between DNA and RNA?
These notes are perfect for quickly comparing DNA and RNA. They highlight the fundamental differences in a concise format, focusing on:
- Sugar: Deoxyribose in DNA vs. Ribose in RNA.
- Nitrogenous Bases: DNA has Adenine, Guanine, Cytosine, and Thymine. RNA has Adenine, Guanine, Cytosine, and Uracil.
- Structure: DNA is typically a double-stranded helix, while RNA is generally single-stranded.
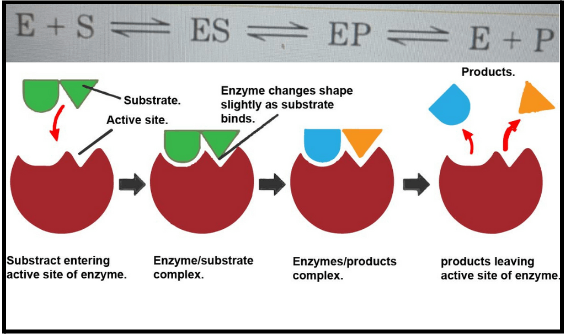
6. What are enzymes, and how do these notes summarise their classification?
The notes define enzymes as biological catalysts, almost always proteins, that speed up metabolic reactions. They summarise the six major classes of enzymes as per the IUB system:
- Oxidoreductases: Catalyse oxidation-reduction reactions.
- Transferases: Transfer a functional group.
- Hydrolases: Catalyse hydrolysis.
- Lyases: Remove groups from substrates, leaving double bonds.
- Isomerases: Catalyse isomerisation changes within a single molecule.
- Ligases: Join two molecules together.
7. Why are lipids, despite their small molecular weight, considered part of the acid-insoluble fraction? How do the notes clarify this?
This is a key conceptual exception explained clearly in the notes. While lipids have a molecular weight of less than 800 Da (making them micromolecules), they are found in the acid-insoluble fraction because they are a major component of cell membranes. During tissue grinding, these membranes break into vesicles which are not water-soluble. These vesicles get separated along with the true macromolecules (like proteins and nucleic acids) in the pellet.
8. Beyond definitions, how do these notes help in understanding the relationship between the four levels of protein structure?
The notes go beyond simple definitions by showing how each level builds upon the previous one. They explain that the primary structure (the amino acid sequence) dictates how the protein will fold into the secondary structures (α-helix, β-sheet). These, in turn, arrange into a stable tertiary structure. For many proteins, the final functional form is the quaternary structure, which involves the assembly of multiple polypeptide chains. This helps in understanding that a protein's function is entirely dependent on its 3D conformation, which originates from its primary sequence.
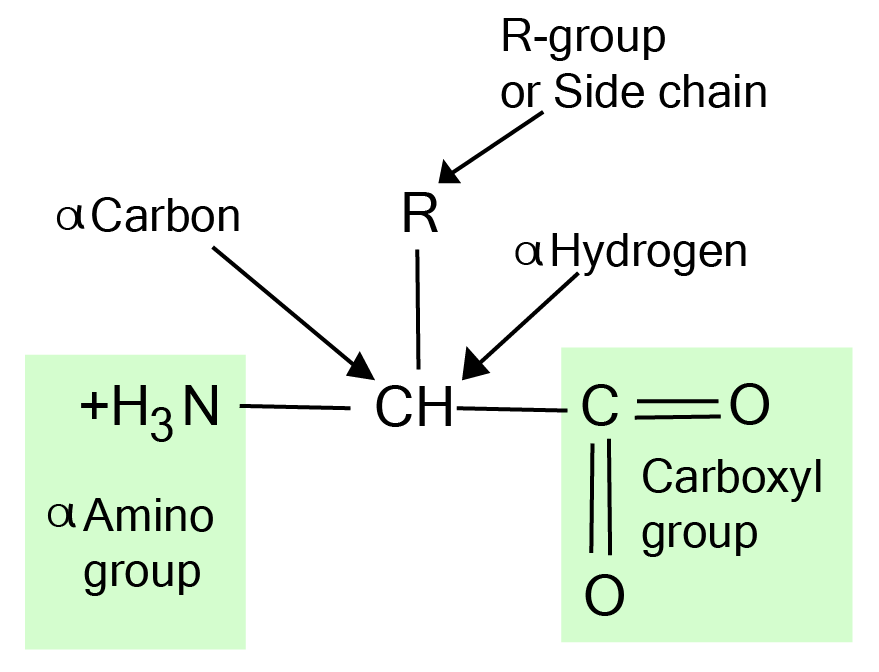
9. What is the 'Zwitterion' concept for amino acids, and how do the notes simplify this for revision?
The revision notes simplify the Zwitterion concept by explaining it as the state of an amino acid in a solution of a particular pH. An amino acid has both an acidic carboxyl group (-COOH) and a basic amino group (-NH2). A zwitterion is a neutral molecule with both a positive and a negative electrical charge at different locations within that molecule. The notes clarify that this dipolar ionic form is how amino acids exist in solutions, allowing them to act as buffers.
10. How do these notes explain the concept of a 'living state' in relation to metabolism for Class 11 students?
The notes explain that the living state is a non-equilibrium steady-state. Living organisms constantly have chemical reactions (metabolism) occurring, where molecules are being made and broken down. This means the concentrations of biomolecules are not at equilibrium. The notes summarise that this constant flow of energy and matter, which prevents the system from reaching equilibrium, is the hallmark of life.