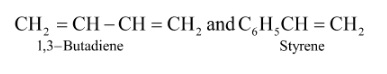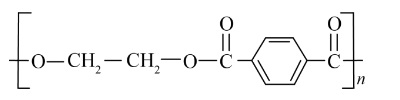Class 12 Chemistry Chapter 15 Summary Notes PDF Download
FAQs on Polymers Class 12 Chemistry Chapter 15 CBSE Notes - 2025-26
1. What is the core concept of a polymer and the process of polymerisation for a quick revision?
A polymer is a high molecular mass substance, also called a macromolecule, which is formed by the chemical combination of a large number of simple molecules called monomers. The process through which these monomers combine to form a polymer is known as polymerisation. For example, ethene monomers polymerise to form the polymer polythene.
2. How are polymers classified based on their source of origin according to the NCERT syllabus?
Based on their source, polymers are classified into three main types:
- Natural Polymers: These are found in nature, in plants and animals. Examples include proteins, cellulose, starch, and natural rubber.
- Semi-synthetic Polymers: These are derived from natural polymers through chemical modifications. An example is cellulose acetate (rayon).
- Synthetic Polymers: These are completely man-made polymers synthesised in laboratories. Examples include nylon, polythene, and bakelite.
3. What is the key difference between addition and condensation polymerisation?
The key difference lies in how the monomers combine. In addition polymerisation, monomers (usually with double or triple bonds) add to one another in a chain reaction without the loss of any small molecules. In condensation polymerisation, monomers combine with the elimination of a small molecule like water, ammonia, or alcohol. This is also known as step-growth polymerisation.
4. How do intermolecular forces influence the properties of different polymers like elastomers and fibres?
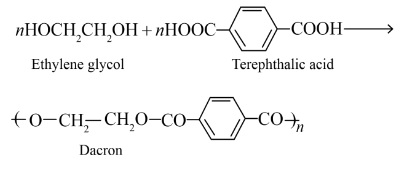
Intermolecular forces are crucial for a polymer's physical properties. Elastomers, like Buna-S, have the weakest intermolecular forces, which allows the polymer chains to stretch under force and return to their original shape. In contrast, fibres, like Terylene or Nylon, have very strong intermolecular forces (such as hydrogen bonding), which leads to high tensile strength and minimal stretching. Thermoplastics have forces intermediate between these two.
5. For a quick recap, what do the numbers in the names 'Nylon-6,6' and 'Nylon-6' signify?
The numbers in the name of a nylon polymer indicate the number of carbon atoms in its monomer units.
- In Nylon-6,6, the '6,6' signifies that it is formed from two different monomers, each containing six carbon atoms: hexamethylenediamine (6 carbons) and adipic acid (6 carbons).
- In Nylon-6, the single '6' indicates that it is formed from a single type of monomer that contains six carbon atoms, which is caprolactam.
6. Why is the process of vulcanisation essential for improving the properties of natural rubber?
Natural rubber is soft, sticky, and has low tensile strength. Vulcanisation is a chemical process where natural rubber is heated with sulphur. The sulphur atoms form cross-links between the long polymer chains of isoprene. These cross-links make the rubber harder, tougher, and more elastic, improving its tensile strength and resistance to temperature changes, making it suitable for practical applications like tyres.
7. How do thermoplastic and thermosetting polymers differ in their structure and reusability?
Thermoplastic polymers consist of long, linear chains with weak intermolecular forces holding them together. Upon heating, these forces weaken, and the polymer can be softened, moulded, and then hardened on cooling. This process is reversible, making them reusable (e.g., PVC, Polythene). In contrast, thermosetting polymers form an extensive 3D cross-linked structure upon heating. These strong covalent cross-links are permanent, so the polymer becomes hard and infusible and cannot be reshaped, making it non-reusable (e.g., Bakelite, Melamine).
8. What is the concept of biodegradable polymers, and can you name a key synthetic example from the Class 12 syllabus?
Biodegradable polymers are polymers that can be decomposed by microbial action over a period of time, helping to reduce plastic waste. While many natural polymers are biodegradable, some synthetic ones are also designed to be. A key example from the NCERT syllabus is PHBV (Poly-β-hydroxybutyrate-co-β-hydroxy valerate). It is a copolymer of 3-hydroxybutanoic acid and 3-hydroxypentanoic acid.
9. Can complex natural molecules like proteins and nucleic acids be considered polymers?
Yes, absolutely. Proteins and nucleic acids are excellent examples of natural polymers, often called biopolymers. Proteins are polymers of amino acid monomers linked by peptide bonds. Nucleic acids (DNA and RNA) are polymers of nucleotide monomers. They are generally formed through condensation polymerisation, where a water molecule is eliminated during the formation of each bond.
10. What is the fundamental structural difference between Novolac and Bakelite, given they share the same monomers?
Although both Novolac and Bakelite are phenol-formaldehyde polymers, their structures differ significantly. Novolac is a linear polymer with a straight-chain structure. It is a thermoplastic. Bakelite, on the other hand, is formed when Novolac is heated with more formaldehyde, causing extensive cross-linking between the linear chains. This creates a rigid, three-dimensional network structure, making Bakelite a thermosetting polymer.