What Are the Main Types of Antibodies and How Do They Protect You?
When you catch a cold or flu, you might notice mild swelling around your neck. This happens because your lymph nodes accumulate white blood cells (WBCs) that help fight off invading germs. Among these WBCs are specialised defenders called antibodies, which play a key role in protecting your body from infections. In this article, we will delve into the structure of antibodies, understand their production, explore the types of antibodies and their functions, and highlight how these tiny Y-shaped proteins help maintain our immunity.
Introduction
Antibodies, also known as immunoglobulins (Ig), are specialised proteins produced by B lymphocytes (a type of white blood cell) in response to the presence of antigens. An antigen is any foreign substance—such as bacteria, viruses, toxins, or other pathogens—that triggers an immune response in the body.
Key Points
Antibodies are Y-shaped proteins designed to identify and neutralise specific antigens.
They circulate in bodily fluids, including blood and lymph, and can also be present in secretions like saliva and breast milk.
Once an antibody binds to its corresponding antigen, it can mark the pathogen for destruction, neutralise its harmful effects, or initiate a cascade of immune responses.
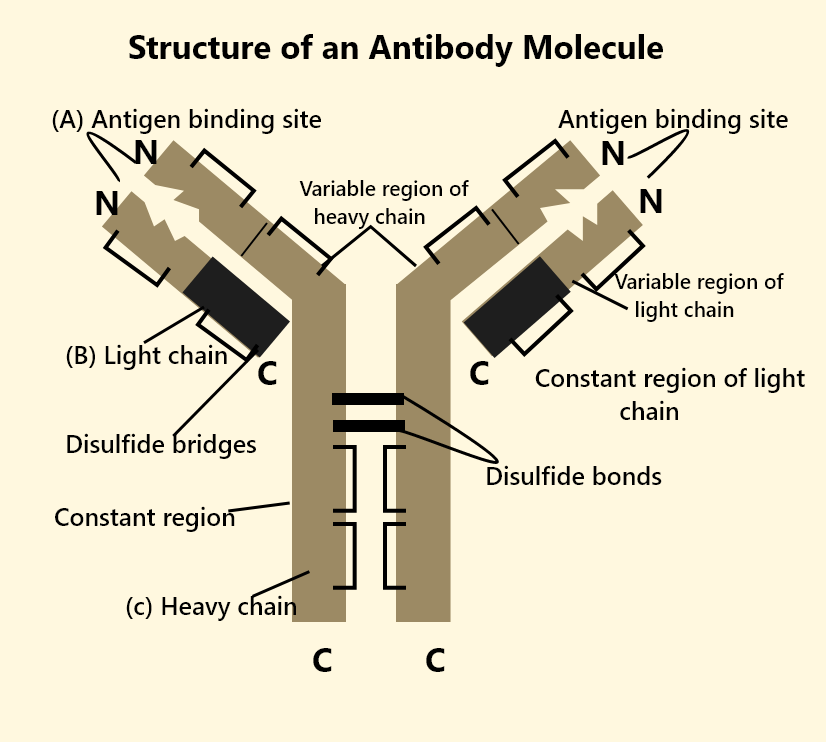
Structure of Antibody Molecule
Understanding the structure of antibody molecules is crucial to appreciate antibody structure and function. Each antibody typically has a Y-shaped configuration made of four polypeptide chains:
Two Heavy (H) Chains: These are longer polypeptide chains that determine the class (isotype) of the immunoglobulin.
Two Light (L) Chains: These are shorter and help form the antigen-binding sites.
Immunoglobulin Structure
When we talk about immunoglobulin structure, the chains are held together by disulphide bonds and other non-covalent interactions. The main regions of an antibody molecule are:
Variable (V) Region: Present at the tips of the Y shape, forming the antigen-binding sites. This region differs from one antibody to another, enabling specificity for unique antigens.
Constant (C) Region: The stem of the Y shape and portions of the arms that do not vary significantly among antibodies of the same class. This region determines how the antibody interacts with other components of the immune system.
Key Functional Segments
Fab (Fragment Antigen-Binding) Region: The two ‘arms’ of the Y that bind specifically to the antigen.
Fc (Fragment Crystallisation) Region: The ‘stem’ that interacts with various immune cells (like macrophages) and complements proteins, thus triggering a wider immune response.
By studying the structure of antibodies, we learn how the molecule locks onto an antigen and instigates its elimination.
Types of Antibodies and Their Functions
One of the most important aspects of immunoglobulin structure is that it allows for different classes (isotypes) of antibodies. Each class has distinct roles and unique properties. Let’s explore the types of antibodies and their functions:
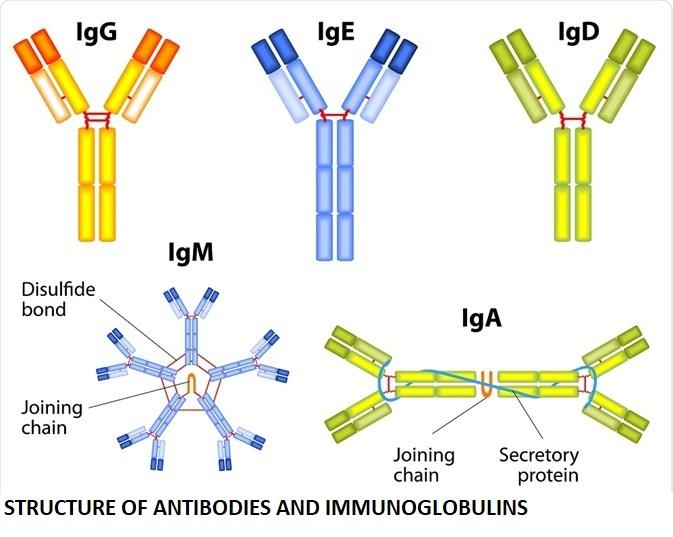
1. IgM
First Responder: IgM is produced first upon initial exposure to an antigen.
Pentameric Form: Usually circulates as five units joined together, making it effective at clumping pathogens.
Functions: Facilitates agglutination (clumping of antigens) and activates the complement system, thus enhancing pathogen destruction.
2. IgG
Most Abundant: Constitutes about 80% of the total antibody content in the blood.
Crosses Placenta: IgG is the only antibody that can cross the placenta, providing foetal immunity.
Functions: Neutralises toxins, supports phagocytosis, and offers long-term protection.
3. IgA
Secretory Antibody: Predominantly found in saliva, tears, breast milk, and intestinal fluids.
Dimeric in Secretions: Often present as two units linked together, especially in bodily secretions.
Functions: Acts as the first line of defence on mucosal surfaces, preventing the attachment of pathogens to epithelial cells.
4. IgD
Receptor on B Cells: Found mainly bound to the surface of B lymphocytes.
Functions: Plays a key role in the activation and regulation of B cells.
5. IgE
Least Abundant: Accounts for a very small fraction of antibodies in circulation.
Allergic Reactions: Responsible for immediate hypersensitivity reactions (e.g., pollen allergies).
Functions: Binds to allergens and triggers histamine release from mast cells, causing inflammation.
When learning about the types of antibodies and their functions, remember that each class is essential in different defence strategies, ensuring a comprehensive immune response.
Antibody Production and Mechanism of Action
Primary Response
When a pathogen enters the body for the first time:
Macrophages or other antigen-presenting cells engulf the pathogen and present antigen fragments on their surface.
Helper T Cells recognise these fragments and activate B Cells.
Plasma B Cells produce antibodies with a specific region (paratope) designed to bind to the pathogen’s unique region (epitope).
Also Read: Difference Between T Cells and B Cells
Secondary Response
Once the infection subsides, some Memory B Cells remain in the body.
If the same pathogen re-enters, these memory cells promptly produce large quantities of antibodies, rapidly neutralising the threat.
This efficient response underscores the importance of antibody structure and function in immune memory, explaining how vaccines work by training the immune system to respond faster.
Difference Between Antigen and Antibody
Understanding the distinction between antigens and antibodies provides clarity on their roles:
Read More: Difference Between Active and Passive Immunity
Additional Insights: Monoclonal vs. Polyclonal Antibodies
To enrich our understanding beyond the basics:
Monoclonal Antibodies
Produced by identical immune cells that are clones of a unique parent cell.
Recognise a single epitope on an antigen.
Widely used in diagnostics (e.g., pregnancy tests) and therapeutics (e.g., targeted cancer therapy).
Polyclonal Antibodies
Generated by multiple B cell clones in the body.
Recognise different epitopes on the same antigen.
Highly useful in research where strong detection of a pathogen or protein is needed.
This added dimension highlights how the structure of the antibody molecule and its generation can be harnessed for advanced medical and research applications.
Clinical Applications and Unique Facts
Antibody Testing: Diagnostic tests (like ELISA) detect specific antibodies against pathogens (e.g., HIV, SARS-CoV-2), confirming exposure or infection.
Therapeutic Antibodies: Monoclonal antibodies are designed to treat diseases such as rheumatoid arthritis, certain cancers, and autoimmune conditions.
ABO Blood Grouping: Antibodies (Anti-A, Anti-B) are central to determining blood groups and ensuring compatibility in blood transfusions.
Swelling of Lymph Nodes: During infections, the body produces more B cells and antibodies, leading to enlarged lymph nodes commonly felt around the neck or underarms.
These unique applications further emphasise why a detailed grasp of antibody structure and function is so valuable in both everyday health and specialised medicine.
Related Links:


FAQs on Antibodies: Structure, Types, and Immune Functions Simplified
1. What is an antibody in simple terms?
An antibody, also known as an immunoglobulin (Ig), is a Y-shaped protein produced by the body's immune system in response to a foreign substance called an antigen. Its primary job is to identify and neutralize harmful pathogens like bacteria and viruses, playing a vital role in the body's defence mechanism.
2. What are the main parts of an antibody's Y-shaped structure?
An antibody molecule is made of four polypeptide chains: two identical heavy chains and two identical light chains. These chains form a 'Y' shape with distinct regions:
- Antigen-binding sites (Fab region): Located at the tips of the 'Y's arms, this variable region specifically recognizes and binds to a unique antigen.
- Constant region (Fc region): The stem of the 'Y', which interacts with other immune cells and proteins to trigger a larger immune response.
3. What are the five main classes of antibodies and their primary functions?
Humans have five major classes, or isotypes, of antibodies, each with a specific role:
- IgG (Immunoglobulin G): The most abundant antibody in the blood. It provides long-term immunity and is the only antibody that can cross the placenta to protect a foetus.
- IgM (Immunoglobulin M): The first antibody produced during an initial infection. It is very effective at activating other parts of the immune system.
- IgA (Immunoglobulin A): Found in mucosal secretions like saliva, tears, and breast milk. It protects body surfaces from pathogens.
- IgE (Immunoglobulin E): Involved in allergic reactions and defence against parasitic worms.
- IgD (Immunoglobulin D): Found on the surface of B-cells; its exact function is less understood but is thought to help activate the B-cell response.
4. How do antibodies help the body fight off infections?
Antibodies fight infections through several key mechanisms:
- Neutralization: They bind to antigens on a pathogen, blocking it from entering or harming body cells.
- Opsonization: They coat the pathogen, marking it for destruction by other immune cells like phagocytes.
- Complement Activation: The binding of antibodies to a pathogen can trigger the complement system, a cascade of proteins that helps to destroy the invader.
5. What is the difference between an antigen and an antibody?
The main difference lies in their roles. An antigen is any foreign substance (like a virus, bacterium, or pollen) that triggers an immune response. An antibody is the protein that the immune system produces to specifically recognize and fight that antigen. In short, antigens are the 'invaders', and antibodies are the 'defenders'. For a detailed comparison, you can read about the difference between antigen and antibody.
6. Why is it significant that IgG is the only antibody to cross the placenta?
The ability of IgG to cross the placenta is crucial for providing passive immunity to the developing foetus. A newborn's immune system is not fully mature, so it cannot produce its own effective antibodies immediately. By receiving IgG from the mother, the baby is born with a pre-made defence against many common pathogens, protecting it during the first few critical months of life until its own immune system develops.
7. How does the body know how to make the right antibody for a pathogen it has never seen before?
The body achieves this through a process called clonal selection. The immune system contains millions of B-cells (a type of lymphocyte), each capable of producing a unique antibody. When a new pathogen enters the body, the B-cell with the corresponding antibody receptor binds to the pathogen's antigen. This binding event activates the B-cell, causing it to rapidly multiply (clone itself) and differentiate into plasma cells, which then mass-produce the exact antibody needed to fight that specific infection.
8. Are antibodies the same as antibiotics?
No, they are fundamentally different. Antibodies are proteins naturally produced by your immune system to target specific pathogens, including viruses and bacteria. In contrast, antibiotics are drugs (often derived from microorganisms) that are used to kill or inhibit the growth of bacteria only; they have no effect on viruses.










