




Concepts of Biomolecules for JEE Main Chemistry
Every living entity, from plants to mammals, is built up of small building components called cells. They are the tiniest fundamental units that make up our bodies, and they are made up of a variety of inorganic and organic chemicals. All carbon-containing substances found in living stuff are considered biomolecules.
Biomolecules are an important part of the JEE Main syllabus. We've covered all of the main aspects of biomolecules in this article, including what is biomolecules, types of biomolecules, the structure of biomolecules, previous year questions, practice questions, etc. This extensive biomolecules article will help you gain an edge over your competitors.
JEE Main Chemistry Chapters 2026
Important Topics of Biomolecules Chapter
Chirality
Glucose
Anomers
Cellulose
Sucrose
Fructose
Optical isomerism
Pyranose ring
Tollen’s Test
Biomolecules Important Concept for JEE Main
What are Biomolecules?
Biomolecules are organic chemicals that make up the very backbone of a cell in living creatures. Carbohydrates, lipids, and proteins, for example, could all be present in various quantities. All of these biomolecules serve important functions in the body and are produced there. Some examples of biomolecules are DNA, RNA, cellulose, and glucose.
While organic molecules can be made up of straight-chain carbons, they can also be made up of cyclic rings, branched chains, or a combination of these. They differ in terms of characteristics, chemical properties, and structures, which leads to differences in physical attributes like boiling and melting points, as well as water solubility. Biomolecules can be classified as hydrophilic or hydrophobic depending on their affinity for water.
Major Structural Elements of Biomolecules
The majority of biomolecules are organic compounds made up of only four components. The major structural elements of biomolecules are oxygen, carbon, hydrogen, and nitrogen. They account for 96 percent of the mass of the human body. However, several additional elements, such as biometals, are present in trace levels.
Classification of Biomolecules
Make sure you understand organic compounds and their properties before moving on to the next section of this biomolecule article. This will make it easier for you to comprehend the next things. The several types of biomolecules are discussed here.
Carbohydrates, proteins, nucleic acids, and lipids are the four main types of biomolecules. From these, the most abundant biomolecule on earth is carbohydrates.
1. Carbohydrates: Carbohydrates are polysaccharides that are the end products of the majority of our body's metabolic processes. They are the foundations of our metabolic apparatus, and their molecular structure is made up of several monosaccharides stacked on top of one another. Carbohydrates are abundant in most living cells, and it is safe to claim that these biomolecules represent the origin of life on our planet.
For example, cellulose is an essential component of plant cells, and it is normally stored as starch. Glucose, on the other hand, is the end product of photosynthesis, the process by which plants convert sunlight into food. Monosaccharides, disaccharides, and oligosaccharides are the three main types of saccharides. They're divided into groups based on the number of sugar molecules they contain.
Sucrose, cellulose, fructose, glucose, and dextrose are some of the most common sugars we encounter on a regular basis. You'll also learn about the three fundamental types of sugar-containing carbohydrates, namely monosaccharides, oligosaccharides, and disaccharides, in these biomolecules Class 12 notes.
Monosaccharides: They are the most basic carbohydrates and cannot be further hydrolyzed. They are present in the general chemical formulae of (CH2O)n. Ribose and glucose are examples of monosaccharides.
Oligosaccharides: These are complicated carbohydrates that break down into two to ten monosaccharide subunits. They are then subdivided into other categories. Stachyose and raffinose are two examples.
Disaccharides: Disaccharides are carbohydrates that break down into two monosaccharide molecules when hydrolyzed. Sucrose, for example, produces fructose and glucose. Maltose, on the other hand, releases two molecules of glucose when hydrolyzed.
2. Proteins: Proteins are organic substances that are abundant in our meals and are made up of amino acids. They are made up of long-chain monomers joined together by polypeptide bonds. As a result, proteins are also known as polypeptides.
3. Lipids: Fats, oils, steroids, phospholipids, and glycerol are all examples of hydrophobic substances. Lipids can have a variety of structures and properties depending on the ingredients. Fatty acids, for example, are composed of a single carboxyl group attached to a variable group or R. Saturated or unsaturated fatty acids can be found in these foods.
Furthermore, some lipids may contain phosphorus groups that are linked to the organic groups. Phospholipids are another name for them. Phospholipids are the fundamental components of a cell's plasma membrane.
4. Nucleic Acids: The smallest fundamental pieces of our genetic information, often known as genes, are made up of DNA and RNA building blocks. These are a combination of nitrogenous bases, sugar molecules, and a phosphate group that make up our bodies' genetic material.
Purines and pyrimidines are examples of heterocyclic compounds. Xanthine, caffeine, and nitrogenous bases like guanine and adenine are examples of purines. The product of nitrogenous bases forming chemical bonds with sugar molecules is known as a nucleoside. Nucleotides are formed when these molecules interact with phosphate groups (RNA, DNA).
Chemical Constitution of DNA and RNA:
DNA (Deoxyribonucleic Acid):
Sugar: DNA contains deoxyribose, a five-carbon sugar.
Bases: DNA consists of four nitrogenous bases: adenine (A), thymine (T), cytosine (C), and guanine (G).
Phosphate Group: DNA also includes a phosphate group.
In DNA, the nitrogenous bases pair specifically: A with T and C with G. These base pairs form the rungs of the DNA double helix, connected by sugar-phosphate backbones. DNA is a double-stranded molecule, and the sequence of these base pairs encodes genetic information.
RNA (Ribonucleic Acid):
Sugar: RNA contains ribose, another five-carbon sugar.
Bases: RNA includes adenine (A), uracil (U), cytosine (C), and guanine (G).
Phosphate Group: RNA, like DNA, also contains a phosphate group.
Unlike DNA, RNA is typically single-stranded, but it can form complex secondary structures due to base pairing. Instead of thymine, RNA contains uracil, which pairs with adenine.
Biological Functions of Nucleic Acids:
DNA Functions:
Genetic Storage: DNA serves as the primary repository for an organism's genetic information. The sequence of base pairs in DNA encodes the instructions for building and maintaining an organism.
Replication: DNA replication is a process in which DNA molecules make copies of themselves. This is crucial for cell division, growth, and repair.
Transcription: In transcription, a complementary RNA molecule is synthesized based on a specific segment of DNA. This RNA, called messenger RNA (mRNA), carries genetic information from the DNA to the ribosomes for protein synthesis.
Protein Synthesis: DNA provides the instructions for building proteins. This process, known as translation, takes place on ribosomes and involves multiple types of RNA.
RNA Functions:
Messenger RNA (mRNA): mRNA carries the genetic code from DNA to ribosomes, where it is translated into a specific protein.
Transfer RNA (tRNA): tRNA molecules bring amino acids to the ribosomes during protein synthesis. Each tRNA has an anticodon that pairs with a specific mRNA codon.
Ribosomal RNA (rRNA): rRNA is a component of ribosomes, the cellular structures where protein synthesis occurs. It helps catalyze the formation of peptide bonds between amino acids.
Regulatory RNA: Several types of regulatory RNA molecules, such as microRNA (miRNA) and small interfering RNA (siRNA), play roles in post-transcriptional gene regulation by interfering with the translation of specific mRNA molecules.
Biological Significance:
Nucleic acids are essential for the storage, transmission, and expression of genetic information.
They serve as the molecular blueprints for the construction and regulation of all cellular components.
Understanding the structure and function of nucleic acids is fundamental to grasping genetics, molecular biology, and biotechnology.
5. Vitamins: Classification and Functions
Vitamins are organic compounds that are essential for the proper functioning of our bodies. They play a wide range of roles, from supporting growth and development to maintaining overall health. Understanding the classification and functions of vitamins is essential for JEE Main students, as it provides insights into the biochemical processes vital for life.
Classification of Vitamins:
Vitamins are classified into two main categories based on their solubility in water or fat. These categories are water-soluble vitamins and fat-soluble vitamins.
Water-Soluble Vitamins:
Vitamin C (Ascorbic Acid):
Function: Vitamin C is crucial for the growth, development, and repair of all body tissues. It's involved in numerous bodily functions, including collagen formation, iron absorption, immune system support, and antioxidant protection.
Sources: Citrus fruits (oranges, lemons, grapefruits), strawberries, kiwi, tomatoes, and bell peppers.
B Vitamins:
B1 (Thiamine):
Function: Thiamine is essential for the metabolism of carbohydrates and plays a vital role in nerve function.
Sources: Whole grains, pork, and legumes.
B2 (Riboflavin):
Function: Riboflavin is involved in energy production, cell growth, and maintaining healthy skin.
Sources: Dairy products, lean meats, and green leafy vegetables.
B3 (Niacin):
Function: Niacin is crucial for the metabolism of carbohydrates and fats and plays a role in DNA repair.
Sources: Meat, fish, and peanuts.
B5 (Pantothenic Acid):
Function: Pantothenic acid is important for energy production and the synthesis of fatty acids.
Sources: Meat, vegetables, and whole grains.
B6 (Pyridoxine):
Function: Pyridoxine is involved in amino acid metabolism and neurotransmitter synthesis.
Sources: Meat, fish, and chickpeas.
B7 (Biotin):
Function: Biotin plays a role in fatty acid synthesis and is essential for maintaining healthy hair, skin, and nails.
Sources: Eggs, nuts, and sweet potatoes.
B9 (Folate):
Function: Folate is vital for cell division, DNA synthesis, and the formation of red blood cells.
Sources: Leafy greens, legumes, and fortified grains.
B12 (Cobalamin):
Function: Vitamin B12 is crucial for the formation of red blood cells and neurological function.
Sources: Animal products like meat, fish, and dairy.
Biotin and Pantothenic Acid: These vitamins are essential for energy production, fatty acid synthesis, and maintaining healthy hair, skin, and nails.
Fat-Soluble Vitamins:
Vitamin A (Retinol):
Function: Vitamin A is essential for vision, immune function, and skin health. It also plays a role in cell growth and differentiation.
Sources: Liver, fish, dairy products, and fortified cereals.
Vitamin D (Cholecalciferol):
Function: Vitamin D is vital for calcium absorption, bone health, and immune function.
Sources: Sunlight exposure, fatty fish, and fortified dairy products.
Vitamin E (Tocopherol):
Function: Vitamin E is a potent antioxidant that protects cells from oxidative damage.
Sources: Nuts, seeds, and vegetable oils.
Vitamin K (Phylloquinone):
Function: Vitamin K is essential for blood clotting and bone health.
Sources: Leafy greens, broccoli, and Brussels sprouts.
Functions of Vitamins:
Energy Production: B vitamins play a crucial role in energy metabolism, helping convert food into usable energy.
Antioxidant Protection: Vitamins C and E are powerful antioxidants that neutralize harmful free radicals, protecting cells from damage.
Immune Support: Vitamin C enhances the immune system's response to infections and illnesses.
Skin Health: Vitamin A is essential for healthy skin, vision, and immune function.
Bone Health: Vitamin D aids in calcium absorption and plays a significant role in maintaining strong bones.
Blood Clotting: Vitamin K is necessary for proper blood clotting, preventing excessive bleeding.
DNA Synthesis: Folate (B9) is vital for DNA synthesis and cell division.
Nervous System Function: B vitamins, such as thiamine (B1) and cobalamin (B12), support proper nervous system function.
Cell Growth and Repair: Several vitamins, including vitamin A, support cell growth and tissue repair.
Eye Health: Vitamin A is essential for maintaining good vision, especially in low-light conditions.
Cardiovascular Health: Some B vitamins, like niacin (B3), help maintain healthy cholesterol levels.
Red Blood Cell Formation: Folate (B9) and cobalamin (B12) are essential for red blood cell production.
Importance of Biomolecules
Biomolecules are essential for life since they assist organisms in growing, living, and reproducing. They aid the development of organisms ranging from single cells to sophisticated living beings such as humans by interacting with one another. Because of their shape and structure, they can be used for a variety of purposes.
JEE Main Biomolecules Solved Examples
1. Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six-membered ring compounds) are insoluble in water - Explain.
Ans: Glucose and sucrose, respectively, have five and eight -OH groups. As a result, they're more likely to form hydrogen bonds with water molecules. Because their electronegativity differences are so small, cyclohexane and benzene are non-polar compounds. As a result, they are not water soluble.
Key Points to remember: Although carbon chains are naturally hydrophobic, the introduction of strong electronegative elements causes them to become hydrophilic due to the development of polarity in the molecules.
2. Where does the water present in the egg go after boiling the egg?
Ans: When an egg is cooked, the globular proteins (egg white albumin) coagulate (denature) into a rubbery, intractable mass. As it forms hydrogen bonds with the egg's water, this insoluble mass swallows it all.
Key Points to remember: Protein denaturation occurs at high temperatures.
Solved Problems of Previous Year Question from the Chapter: Biomolecules
1. The helical structure of a protein is formed by which bond?
Options:
(a) H-bond
(b) Peptide bond
(c) Dipeptide bond
(d) None of these
Ans: The correct answer is option a.

Trick: Certain folding patterns are caused by hydrogen bonding between amino groups and carboxyl groups in neighbouring regions of the protein chain.
2. Match the following:
Options:
(a) (i)-(c), (ii)-(a), (iii)-(d), (iv)-(b)
(b) (i)-(c), (ii)-(d), (iii)-(a), (iv)-(b)
(c) (i)-(a), (ii)-(d), (iii)-(c), (iv)-(b)
(d) (i)-(d), (ii)-(b), (iii)-(a), (iv)-(c)
Ans: The correct answer is option a.
Trick: Ascorbic acid protects and maintains the health of cells.
Riboflavin aids in the formation of red blood cells.
Thiamine aids the conversion of carbohydrates into energy in the body's cells.
Pyridoxine is a B vitamin that aids nerve activity.
3. Out of the following isomeric forms of uracil, which one is present in RNA?
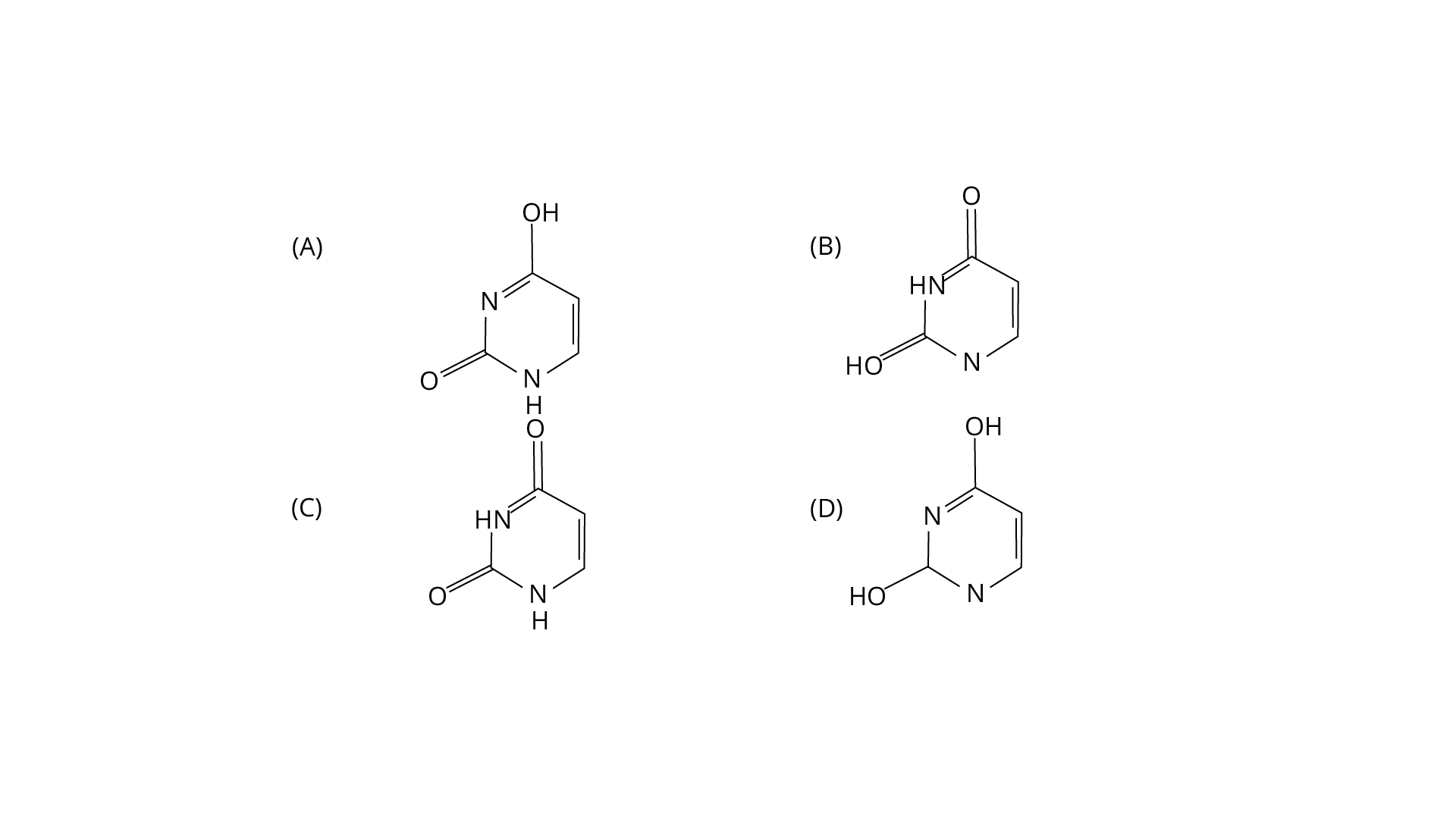
Ans: The correct answer is option c. It is a heterocyclic compound with double bonded two oxygens.
Trick: The chemical formula of uracil is C4H4N2O2.
Practice Questions
1. What products would be formed when a nucleotide from DNA containing
thymine is hydrolysed? (Ans: Thymine β-D-2-deoxyribose and phosphoric acid )
2. What are the hydrolysis products of sucrose? (Ans: Glucose and fructose)
JEE Main Chemistry Biomolecules Study Materials
Here, you'll find a comprehensive collection of study resources for Biomolecules designed to help you excel in your JEE Main preparation. These materials cover various topics, providing you with a range of valuable content to support your studies. Simply click on the links below to access the study materials of Biomolecules and enhance your preparation for this challenging exam.
JEE Main Chemistry Study and Practice Materials
Explore an array of resources in the JEE Main Chemistry Study and Practice Materials section. Our practice materials offer a wide variety of questions, comprehensive solutions, and a realistic test experience to elevate your preparation for the JEE Main exam. These tools are indispensable for self-assessment, boosting confidence, and refining problem-solving abilities, guaranteeing your readiness for the test. Explore the links below to enrich your Chemistry preparation.
Benefits of Using Vedantu for JEE Main Chapter - Biomolecules
Unlock the world of Biomolecules with Vedantu's tailored approach for JEE Main, offering detailed insights, interactive sessions, and real-world applications to ensure comprehensive understanding and success in the exam. Experience structured learning, visual aids, and flexible preparation at your own pace.
Detailed Understanding: Vedantu ensures a comprehensive grasp of Biomolecules, breaking down intricate concepts for in-depth understanding, crucial for JEE Main preparation.
Topic-Wise Coverage: The platform structures Biomolecules topics systematically, simplifying complex ideas and aiding clarity, enhancing your preparation for the JEE Main Exam.
Visual Learning Resources: Vedantu integrates visual aids into Biomolecules lessons, aiding visual learners in understanding molecular structures and enhancing JEE Main exam readiness.
Interactive Sessions: Engage in interactive sessions tailored for Biomolecules, where doubts are clarified in real-time, fostering a deeper understanding essential for JEE Main success.
Practice Questions: Access a diverse range of practice questions specific to Biomolecules, honing your problem-solving skills and preparing you thoroughly for the JEE Main Exam.
Exam-Oriented Preparation: Vedantu aligns its Biomolecules study material with the JEE Main Exam pattern, ensuring a focused and effective approach to meet exam expectations.
Real-Life Applications: Explore the real-life applications of Biomolecules through practical examples, bridging the gap between theory and the real world, a valuable aspect for JEE Main aspirants.
Accessible Learning: Vedantu's platform allows flexibility in Biomolecules preparation for the JEE Main Exam, accommodating your pace and ensuring accessible learning for optimal understanding.
Conclusion
Biomolecules, the fundamental entities of life, are crucial for JEE Main students to comprehend. This chapter delves into the diverse world of carbohydrates, lipids, proteins, nucleic acids, and more, shedding light on their structures and functions. With applications spanning from biology to biotechnology and beyond, biomolecules hold the key to various scientific and medical advancements. Equipping JEE Main students with this knowledge is not only essential for understanding life's molecular intricacies but also for paving the way for future innovations in science and engineering.
Biomolecules Chapter - Chemistry JEE Main

 Share
ShareFAQs on Biomolecules Chapter - Chemistry JEE Main
1. What are the main points to remember while tackling problems involving biomolecules?
Students should practise drawing the ring structures of the biomolecules to remember the linkages between the molecules because most of the answers in biomolecules are based on these linkages.
2. Do questions from the biomolecule chapter come every year in JEE Main?
yes, the biomolecule chapter is one of the important chapters that comes in JEE Main every year. This chapter is considered as a scoring chapter as the topics under this chapter are very less and easy to understand.
3. What is the weightage of this chapter in JEE Main?
Nearly 1-2 questions arise in the exam from this chapter covering about 4 marks which makes about 2% of the total marks.
4. What is biomolecules definition for JEE Mains?
In the context of JEE Main, biomolecules refer to the complex organic molecules present in living organisms. These molecules play critical roles in biological processes and are classified into various categories based on their chemical composition and functions. Biomolecules encompass a wide range of compounds, including carbohydrates, lipids, proteins, nucleic acids, and other smaller organic molecules essential for life processes. Understanding their structures, functions, and interactions is crucial in comprehending the biochemical foundations of life and is often a topic of study in JEE Main chemistry.
5. Give 10 biomolecules examples that are important for JEE Main Exam.
Understanding these biomolecules, their structures, functions, and interactions, is crucial for excelling in the biochemistry section of the JEE Main Exam. Here are ten important biomolecules for the JEE Main Exam:
Glucose: A fundamental carbohydrate and primary source of energy in cells.
DNA (Deoxyribonucleic Acid): The hereditary material carrying genetic information.
RNA (Ribonucleic Acid): Essential for protein synthesis and gene expression.
Proteins: Comprising amino acids, they serve structural, enzymatic, and regulatory functions.
Lipids: Including triglycerides and phospholipids, crucial for energy storage and cell membrane structure.
ATP (Adenosine Triphosphate): Energy currency of the cell, facilitating energy transfer in metabolic processes.
Enzymes: Protein molecules catalyzing biochemical reactions in living organisms.
Hemoglobin: A protein in red blood cells responsible for oxygen transport in the body.
Cholesterol: An essential lipid, vital for cell membrane structure and hormone synthesis.
Glycogen: A carbohydrate stored in the liver and muscles, serving as a short-term energy reserve.




















 Watch Video
Watch Video


