What Happens to Cells in a Hypotonic Solution?
Tonicity refers to a solution's relative solute concentration in comparison to another solution. A hypotonic solution is a type of tonicity. It is one in which the concentration of soluble chemicals outside the cell is less than that inside the cell.
In a hypotonic solution, the solute concretion is always smaller than the cell. There is less solvent because there is a high concentration of solute inside the cells (water).
What is Hypotonic Solution?
Hypo denotes low, hence a hypotonic solution is one that has a higher water content than solute concentration. A solution is a molecularly dispersed mixture of one or more compounds in a sufficient quantity of dissolving solvent. A solute is a dissolved material in a solution. What enters and leaves the cell is regulated and controlled by the cell membrane. A selectively permeable membrane is a membrane that enables certain materials to pass through but not others.
In a hypotonic solution, the solute concretion is always smaller than the cell. There is less solvent because there is a high concentration of solute inside the cells (water).
Osmosis is a process in which water passes through a semipermeable membrane from a high concentration to a low concentration. The fact that the solute concentration is low in this solution implies that the solvent concentration is high. Water flows from the outside to the inside of the cell.
Hypertonic and Hypotonic Solution
A hypertonic solution is one in which the concentration of solutes in the solution is higher than the concentration of the cell in the solution. Saltwater is an example of a hypertonic solution. Whereas a hypotonic solution is a solution that has more water outside the cell. Water then travels into the cell from the solution.
Both animal and plant cells have been shown to be affected by hypertonic and hypotonic solutions. Hypertonic solution has a higher solute content and less water than a cell. There is a net migration of water from inside to outside the cell because the concentration of water is higher within the cell. Osmosis allows water to leave the cell. The loss of water causes the cells in a hypertonic solution to shrink. The animal cell will shrivel up in a hypertonic solution. This is known as crenation. The plant cell becomes less stiff in a hypertonic solution, which is known as plasmolysis.
In a hypotonic solution, The water enters the cell due to high solute concentration inside the cell. The water is more in this hypotonic solution and solute concentrations are low.
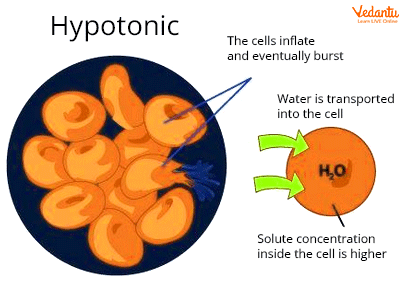
Image: Hypotonic solution
Hypotonic Solution Example
Hypotonic solutions involve distilled and clean water. Hypotonic solutions include 0.45 per cent saline and 5% dextrose in water (this is technically isotonic, but once the dextrose is absorbed, it operates on the body as if it were hypotonic).
When Cells are Placed in a Hypotonic Solution
A hypotonic solution contains a lower concentration of solutes and a higher concentration of solvent. Water fills a cell through osmosis when it is placed in a hypotonic solution. Due to the lack of a cell wall, animal cells enlarge and explode.
During hypotonic solution, the animal cell ruptures. This is called hemolysis. The plant cell would load up and become much stiffer in a hypotonic solution, and this is known as turgor pressure. The plant cell has a cell wall that exerts reverse pressure on the cell membrane.
Administration of Hypotonic Solution
Hypotonic solutions are frequently employed to dilute extracellular fluid and rehydrate cells in individuals with hypertonic fluid imbalances, as well as to treat gastric fluid loss and dehydration caused by severe diuresis. This type of solution contains no calories or other electrolytes, but it does contain free water, salt, and chloride. The cell has a low amount of extracellular solute and wants to shift into the cell to utilise standard osmosis. This promotes cell swelling, which can result in the cell bursting or lysing.
0.45 percent saline (1/2 NS), 0.225 percent saline (1/4 NS), and 0.33 percent saline (1/3 NS) are hypotonic solutions. When a cell is dehydrated and fluids must be reintroduced, hypotonic solutions are used. This happens when a patient develops diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemia.
When we talk about Intravenous (IV) fluids, we usually mean that the water wants to exit the intravascular region and enter the Red Blood Cells (RBCs). The most common cause of giving 0.45 percent salinity is real dehydration, which occurs when the body loses solely water and no electrolytes (this is different from fluid volume deficit, when the body loses both water and electrolytes). The body already has a normal amount of electrolytes in dehydration. Therefore, there is no need to add extra to the IV solution. Only some of the patient's water needs to be refilled.
Conclusion
A hypotonic solution has the feature that it has a low solute concentration as compared to the cell. This causes cell swelling, and this property is used in some clinical trials to treat patients. This type of solution is provided intravenously and helps to regain the normal state of the cell and body. This article helps to understand the properties of the hypotonic solution and its meaning. It has provided all the information regarding hypotonic solutions with examples.


FAQs on Hypotonic Solution Explained for Students
1. What is a hypotonic solution in biology?
In biology, a hypotonic solution is one that has a lower concentration of solutes compared to the fluid inside a cell. This results in a higher water potential outside the cell. Due to osmosis, there is a net movement of water across the semipermeable cell membrane from the solution into the cell.
2. How do hypotonic, hypertonic, and isotonic solutions differ based on solute concentration?
The primary difference lies in the solute concentration relative to a cell's cytoplasm, which dictates the direction of water movement:
Hypotonic Solution: Has a lower solute concentration than the cell. Water moves into the cell.
Hypertonic Solution: Has a higher solute concentration than the cell. Water moves out of the cell.
Isotonic Solution: Has an equal solute concentration to the cell. There is no net movement of water.
3. What happens when an animal cell, such as a red blood cell, is placed in a hypotonic solution?
When an animal cell is placed in a hypotonic solution, water rapidly enters the cell through endosmosis. Because animal cells lack a rigid cell wall, the cell membrane cannot withstand the increasing internal pressure. As a result, the cell will swell and eventually burst, a process known as cytolysis (or hemolysis in the case of red blood cells).
4. Why don't plant cells burst when placed in a hypotonic solution like animal cells do?
Plant cells do not burst in a hypotonic solution because they are protected by a strong, rigid cell wall located outside the plasma membrane. As water enters the cell, the plasma membrane swells and presses against the cell wall. The wall exerts an opposing pressure, called turgor pressure, which prevents further water entry and stops the cell from bursting. Instead, the cell becomes firm or turgid.
5. What are some common examples of hypotonic solutions?
Common examples of hypotonic solutions relative to most living cells include:
Distilled water: It has virtually no solutes and is a classic example of a hypotonic medium.
Freshwater: Ponds, lakes, and rivers are hypotonic environments for the organisms living in them.
0.45% Saline (Half Normal Saline): In a medical context, this solution is hypotonic compared to human blood plasma.
6. What is the significance of turgor pressure, which develops when a plant cell is in a hypotonic solution?
Turgor pressure is vital for plants. It provides structural support to non-woody stems and leaves, helping them stand upright and face the sun for photosynthesis. This internal pressure is also essential for cell growth and expansion. When a plant loses water, it loses turgor pressure, causing the plant to wilt.
7. How do single-celled freshwater organisms like Amoeba survive in their naturally hypotonic environment?
Single-celled organisms like Amoeba survive in hypotonic freshwater through a process called osmoregulation. They possess a specialized organelle called a contractile vacuole. This structure actively collects the excess water that constantly enters the cell via osmosis and then pumps it back out into the environment, preventing the cell from swelling and bursting.
8. In the context of osmosis, does only water move across the cell membrane in a hypotonic solution?
While the most significant effect in a hypotonic solution is the net movement of water into the cell, the cell membrane is selectively permeable, not just permeable to water. It regulates the passage of various substances. However, the difference in water potential is the primary driver of the osmotic process, causing a much larger and faster influx of water compared to the movement of solute particles, which is often restricted or occurs via specific transport channels.










